Abstract
Tricyclohexylphosphanegold(I) n-mercaptobenzoate (n = 2, 3, 4) labelled as 1–3 were previously reported to significantly suppress thioredoxin reductase (TrxR) activities towards ovarian cancer cells, A2780, in vitro. Herein, we explored the role of 1–3 for their apoptosis inducing ability against A2780 cells. 1–3 exhibited IC50 values at 1.19 ± 0.03 µM, 2.28 ± 0.04 μM and 0.78 ± 0.01 μM, respectively, compared to cisplatin at 26.8 ± 0.15 µM. The compounds induced A2780 apoptosis via a caspase-dependent mitochondrion pathway as evidenced by ROS production, cytochrome c release, caspases-3/7, -8, -9 and -10 activation, APAF1 and BAX upregulation as well as BCL2A1 and BCL2 genes’ downregulation. In addition, the death mode of 1–3 was also mediated via death receptor extrinsic pathway manifested by FAS, FASL, FADD, and TNFR1 genes’ upregulation via Human Rt PCR analysis. In addition, 1–3 significantly caused A2780 arrest at S phase, which was associated with the upregulation of TP53, E2F1, RB1 and CDKN1A upregulation and downregulation of CDK1, CDK4, CDC25A and CDC25C genes. Based on these promising results, these phosphanegold(I) thiolate derivatives could act as feasible candidates for further advanced in vivo ovarian cancer studies to develop novel chemotherapeutic agents derived from metal-based agents.
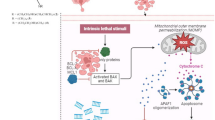
Graphic abstract









Similar content being viewed by others
Availability of data materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- BRCA1:
-
Breast cancer 1
- BRCA2:
-
Breast cancer 2
- FBS:
-
Fetal bovine serum
- DMSO:
-
Dimethylsulfoxide
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PBS:
-
Phosphate buffer saline
- FACS:
-
Fluorescence-activated cell sorting
- DCFH-DA:
-
Dichloro-dihydro-fluorescein diacetate
- PI:
-
Propidium iodide
- FSC:
-
Forward scatter
- SSC:
-
Side scatter
- DNA:
-
Deoxyribonucleic acid
- ROS:
-
Reactive oxygen species
- FLICA:
-
Fluorochrome inhibitor of caspases
- CDK:
-
Cyclin-dependent kinase
- PCR:
-
Polymerase chain reaction
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Nobili S, Mini E, Landini I, Gabbiani C, Casini A, Messori L (2010) Gold compounds as anticancer agents: chemistry, cellular pharmacology, and preclinical studies. Med Res Rev 30:550–580
Higby GJ (1982) Gold in medicine: a review of its use in the West before 1900. Gold Bull 15:130–140
Nardon C, Boscutti G, Fregona D (2014) Beyond platinums: gold complexes as anticancer agents. Anticancer Res 34:487–492
Kostova I (2006) Gold coordination complexes as anticancer agents. Anticancer Agents Med Chem 6:19–32
Azizah AM, Hashimah B, Nirmal K et al (2017) Malaysia National Cancer Registry Report (2012–2016). Ministry of Health, Malaysia
McLemore MR, Miaskowski C, Aouizerat BE, Chen LM, Dodd MJ (2009) Epidemiological and genetic factors associated with ovarian cancer. Cancer Nurs 32:281–290
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2017. National Cancer Institute, Bethesda, MD. https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
Kuchenbaecker KB, Hopper JL, Barnes DR et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416
Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M, González-Fernández A (2011) Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 3(3):3279–3330
Pottier A, Borghi E, Levy L (2014) New use of metals as nanosized radioenhancers. Anticancer Res 34:443–453
Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF (2000) Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695–2701
Donzelli E, Carfi M, Miloso M, Strada A, Galbiati A, Bayssas M, Griffon-Etienne G, Caveletti G (2004) Neurotoxicity of platinum compounds: comparison of the effects of cisplatin and oxaliplatin on the human neuroblastoma cell line SH-SY5Y. J Neurooncol 67:65–73
Boulikas T, Vougiouka M (2003) Cisplatin and platinum drugs at the molecular level. Oncol Rep 10:1663–1682
Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, Tomco D, Dou QP (2010) Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des 16:1813–1825
Abdou HE, Mohamed AA, Fackler JP, Burini A, Galassi R, López-de-Luzuriaga JM, Olmos ME (2009) Structures and properties of gold(I) complexes of interest in biochemical applications. Coord Chem Rev 253:1661–1669
Fernández-Moreira V, Raquel PH, Gimeno MC (2019) Anticancer properties of gold complexes with biologically relevant ligands. Pure Appl Chem 91:247–269
Simon TM, Kunishima DH, Vibert GJ, Lorber A (1979) Cellular antiproliferative action exerted by auranofin. J Rheumatol S5:91–97
Tiekink ER (2003) Phosphinegold(I) thiolates–pharmacological use and potential. Bioinorg Chem Appl 1:53–67
Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello MP (2007) Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Rad Biol Med 42:872–881
Landini I, Lapucci A, Pratesi A, Massai L, Napoli C, Perrone G, Pinzani P, Messori L, Mini E, Nobili S (2017) Selection and characterization of a human ovarian cancer cell line resistant to auranofin. Oncotarget 8:96062–96078
Wani MY, Malik MA (2021) Gold and its complexes in anticancer chemotherapy. Springer, Singapore
Ang KP, Chan PF, Hamid RA (2020) Antiproliferative activity exerted by tricyclohexylphosphanegold(I) n-mercaptobenzoate against MCF-7 and A2780 cell lines: the role of p53 signaling pathways. Biometals. https://doi.org/10.1007/s10534-020-00269-7
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Pocasap P, Weerapreeyakul N, Thumanu K (2019) Alyssin and iberin in cruciferous vegetables exert anticancer activity in HepG2 by increasing intracellular reactive oxygen species and tubulin depolymerization. Biomol Ther (Seoul) 27(6):540–552
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5):479–496
Esmaeili MA, Farimani MM, Kiaei M (2014) Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling pathways, ROS-mediated pathway and mitochondrial dysfunction in hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem 397(1–2):17–31
Wachmann K, Pop C, vanRaam BJ, Drag M, Mace PD, Snipas SJ, Zmasek C, Scwarzenbacher R, Salvesen GS, Riedl SJ (2010) Activation and specificity of human caspase-10. Biochemistry 49:8307–8315
Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol 5(6):a008672
Arora S, Tandon S (2015) DNA fragmentation and cell cycle arrest: a hallmark of apoptosis induced by Ruta graveolens in human colon cancer cells. Homeopathy 104(1):36–47
Wang P, Cui J, Wen J, Guo Y, Zhang L, Chen X (2016) Cisplatin induces HepG2 cell cycle arrest through targeting specific long noncoding RNAs and the p53 signaling pathway. Oncol Lett 12:4605–4612. https://doi.org/10.3892/ol.2016.5288
Wang X, Guo Z (2013) Targeting and delivery of platinum-based anticancer drugs. Chem Soc Rev 42:202–224
Miow QH, Tan TZ, Ye J, Lau JA, Yokomizo T, Thiery JP, Mori S (2015) Epithelial-mesenchymal status renders differential responses to cisplatin in ovarian cancer. Oncogene 34:1899–1907
Zou T, Lum CT, Lok CN, Zhang JJ, Che CM (2015) Chemical biology of anticancer gold(III) and gold(I) complexes. Chem Soc Rev 44(24):8786–8801
Sun C, Mirzadeh N, Guo SX, Li J, Li Z, Bond AM, Zhang J, Bhargava SK (2019) Unprecedented formation of a binuclear Au(II)–Au(II) complex through redox state cycling: Electrochemical interconversion of Au(I)–Au(I), Au(II)–Au(II), and Au(I)–Au(III) in binuclear complexes containing the carbanionic ligand C6F4PPh2. Inorg Chem 58:13999–14004
Mirzadeh N, Reddy TS, Bhargava SK (2019) Advances in diphosphine ligand-containing gold complexes as anticancer agents. Coord Chem Rev 388:343–359
deVos D, Symth DR, Tiekink ER (2002) Cytotoxicity of triorganophosphinegold(I) n-mercaptobenzoates, n-2,3,4. Met Based Drugs 8(6):303–306
Wehr-Candler T, Henderson W (2016) Coordination chemistry of the thiosalicylate ligand. Coord Chem Rev 313:111–155
Tiekink ERT, Henderson W (2017) Coordination chemistry of 3- and 4-mercaptobenzoate ligands: versatile hydrogen-bonding isomers of the thiosalicylate (2-mercaptobenzoate) ligand. Coord Chem Rev 341:19–52
Tyrina A (2019) Interactions of gold thiolates with protein disulfides. BSc Honors College. 531
Pham XH, Hahm E, Huynh KH, Son BS, Kim HM, Jeong DH, Jun BH (2019) 4-Mercaptobenzoic acid labeled gold-silver-alloy-embedded silica nanoparticles as an internal standard containing nanostructures for sensitive quantitative thiram detection. Int J Mol Sci 20:4841. https://doi.org/10.3390/ijms20194841
Zheng K, Setyawati MI, Leong DT, Xie J (2020) Overcoming bacterial physical defenses with molecule-like ultrasmall antimicrobial gold nanoclusters. Bioact Mater 6(4):941–950
Gandin V, Fernandes AP, Rigobello MP, Dani B, Sorrentino F, Tisato F, Bjornstedt M, Bindoli A, Sturaro A, Rella R, Marzano C (2007) Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem Pharmacol 79:90
Abás E, PenaMartínez R, Aguirre-Ramírez D, Rodríguez Diéguez A, Laguna M, Grasa L (2020) New selective thiolate gold(i) complexes inhibit the proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans 49:1915–1927
Florea AM, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 3(1):1351–1371
Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 1863:2977–2992
Jin S, Zhou F, Katirai F, Li PL (2011) Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxid Redox Signal 15(4):1043–1083
Shalini S, Dorstyn L, Dawar S, Kumar S (2015) Old, new and emerging functions of caspases. Cell Death Differ 22:526–539
Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH (2013) Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 14:32. https://doi.org/10.1186/1471-2121-14-32
Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A (2018) How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ 25(1):104–113. https://doi.org/10.1038/cdd.2017.169
Diwanji N, Bergmann A (2018) An unexpected friend – ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Semin Cell Dev Biol 80:74–82
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122(4):437–441
Indran IR, Tufo G, Pervaiz S, Brenner C (2011) Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta Bioenerget 1807:735–745
Zou H, Li Y, Liu X, Wang X (1999) An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274:11549–11556
Chandel NS, Thompson CB, Robey RB, Hay N (2004) Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 16:819–830
Clarke PR, Allan LA (2009) Cell-cycle control in the face of damage–a matter of life or death. Trends Cell Biol 19:89–98
Pena-Blanco A, Garcia-Saez AJ (2018) Bax, Bak and Beyond – mitochondrial performance in apoptosis. FEBS J 285(3):416–431
Ott I, Qian X, Xu Y, Kubutat D, Will J et al (2009) A gold(I) phosphine complex containing naphthalimide ligand functions as a TrxR inhibiting antiproliferative agent and angiogenesis inhibitor. J Med Chem 52:763–770
Caruso F, Villa R, Rossi M, Pettinari C, Paduano F, Pennati M, Daidone MG, Zaffaroni N (2007) Mitochondria are primary targets in apoptosis induced by the mixed phosphine gold species chlorotriphenylphosphine-1,3- bis (diphenylphosphino) propanegold(I) in melanoma cell lines. Biochem Pharmacol 73(6):773–781
Day TW, Huang S, Safa AR (2008) c-FLIP knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and caspase-9-dependent apoptosis in breast cancer cells. Biochem Pharmacol 76(12):1694–1704
Li B, Gao Y, Rankin GO, Rojanasakul Y, Cutler SJ, Tu Y, Chen YC (2015) Chaetoglobosin K induces apoptosis and G2 cell cycle arrest through p53-dependent pathway in cisplatin-resistant ovarian cancer cells. Cancer Lett 356(2):418–433
Horn S, Hughes MA, Schilling R, Sticht C, Tenev T, Ploesser M, Meier P, Sprick M, MacFarlane M, Leverkus M (2017) Caspase-10 negatively regulates caspase-8-mediated cell death, switching the response to CD95L of NF-kB activation and cell survival. Cell Rep 19(4):785–797
Jung EB, Lee SC (2014) Baicalein attenuates proteasome inhibition-induced apoptosis by suppressing the activation of the mitochondrial pathway and the caspase-8- and Bid-dependent pathways. Eur J Pharmacol 730:116–124
Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, Baumgartner F, Logette E, Tschopp J, Villunger A (2009) Caspase-2 activation in the absence of piddosome formation. J Cell Biol 185:291–303
Shi J, Shen HM (2008) Critical role of Bid and Bax in indirubin-3′-monoxime-induced apoptosis in human cancer cells. Biochem Pharmacol 75:1729–1742
Jamaludin NS, Goh ZJ, Cheah YK, Ang KP, Sim JH, Khoo CH, Fairuz ZA, Halim SN, Ng SW, Seng HL, Tiekink ER (2013) Phosphanegold(I) dithiocarbamates, R3PAu[SC(=S)N((i)Pr)CH2CH2OH] for R = Ph, Cy and Et: role of phosphane-bound R substituents upon in vitro cytotoxicity against MCF-7R breast cancer cells and cell death pathways. Eur J Med Chem 67:127–141
Ooi KK, Yeo CI, Ang KP, Akim AM, Cheah YK, Halim SN, Seng HL, Tiekink ER (2015) Phosphanegold(I) thiolates, Ph3PAu[SC(OR)=NC6H4Me-4] for R = Me, Et and iPr, induce apoptosis, cell cycle arrest and inhibit cell invasion of HT-29 colon cancer cells through modulation of the nuclear factor-κB activation pathway and ubiquitination. J Biol Inorg Chem 20:855–873
Sabol SL, Li R, Lee TY, Abdul-Khalek R (1998) Inhibition of apoptosis-associated DNA fragmentation activity in nonapoptotic cells: the role of DNA fragmentation factor-45 (DFF45/ICAD). Biochem Biophys Res Comm 253:151–158
Wu Z, Wu Y, Qin Y, Li X (2014) Influences of sorting and cryopreservation on the mitochondrial membrane potential (MMP) and phosphatidylserine (PS) externalization in bovine sperm. Livest Sci 168:177–182
Marino G, Kroemer G (2013) Mechanisms of apoptotic phosphotidylserine exposure. Cell Res 23:1247–1248
Halliwell B (2012) Free radicals and antioxidants: updating a personal view. Nutr Rev 70(5):257–265
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310
Schreiber M, Kolbus A, Piu F, Szabowski A, Möhle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF (1999) Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev 13(5):607–619
Zhao R, Choi BY, Lee MH, Bode AM, Dong Z (2016) Implications of genetic and epigenetic alterations of CDKN2A (p16INK4a) in cancer. EBioMedicine 8:30–39
Wiese C, Rudolph JH, Jakob B, Fink D, Tobias F, Blanttner C, Taucher-Scholz G (2012) PCNA-dependent accumulation of CDKN1A into nuclear foci after ionizing irradiation. DNA Repair 11:511–521
Longworth MS, Dyson NJ (2010) pRB, a local chromatin organizer with global possibilities. Chromosoma 119(1):1–11
Chen R, Liu S, Ye H, Li J, Du Y, Chen L et al (2015) Association of p53 rs1042522, MDM2 rs2279744, and p21 rs1801270 polymorphisms with retinoblastoma risk and invasion in a Chinese population. Sci Rep 5:13300
Lei ST, Shen F, Chen JW, Feng JH, Cai WS, Shen L, Hui ZW, Xu B (2016) MiR-639 promoted cell proliferation and cell cycle in human thyroid cancer by suppressing CDKN1A expression. Biomed Pharmacother 84:1834–1840
Julian L, Palander O, Seifried L et al (2008) Characterization of an E2F1-specific binding domain in pRB and its implications for apoptotic regulation. Oncogene 27:1572–1579
Röhrs S, Kutzner N, Vlad A, Grunwald T, Ziegler S, Müller O (2009) Chronological expression of Wnt target genes Ccnd1, Myc, Cdkn1a, Tfrc, Plf1 and Ramp3. Cell Biol Int 33:501–508
Brügger D, Brischwein K, Liu C, Bader P, Niethammer D, Gekeler V, Beck JF (2002) Induction of drug resistance and protein kinase C genes in A2780 ovarian cancer cells after incubation with antineoplastic agents at sublethal concentrations. Anticancer Res 22(6C):4229–4232
Acknowledgements
This research was funded by Ministry of Higher Education, Malaysia, grant number UM.C/HIR-MOHE/SC/03 and UM.C/HIR-MOHE/SC/12. We thanked Prof Edward R.T. Tiekink for providing the compounds and Universiti Malaya for providing the funding under High Impact Research Schemes and collaborated with us by providing the compounds and the funding. We also thanked Prof Dr Noor Saadah Abd Rahman, the Deputy Vice Chancellor (Research and Innovation) at the University of Malaya for giving us the consent to publish the data.
Funding
This research was funded by Ministry of Higher Education, Malaysia, grant number UM.C/HIR-MOHE/SC/03 and UM.C/HIR-MOHE/SC/12.
Author information
Authors and Affiliations
Contributions
AKP performed the experiments and wrote the original draft. CPF contributed to experimental studies and data collection. RAH supervised the project, validated the data analysis, edited, revised and submitted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ang, K.P., Chan, P.F. & Hamid, R.A. Induction of apoptosis on ovarian adenocarcinoma cells, A2780 by tricyclohexylphosphanegold (I) mercaptobenzoate derivatives via intrinsic and extrinsic pathways. J Biol Inorg Chem 26, 833–853 (2021). https://doi.org/10.1007/s00775-021-01892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01892-6