Abstract
A novel cytosensor was constructed for the ultrasensitive detection and nondestructive release of circulating tumor cells (CTCs) by combining Au nanoparticles-loaded two-dimensional bimetallic PdMo (2D Au@PdMo) nanozymes and electrochemical reductive desorption. The 2D Au@PdMo nanozymes possessed high-efficiency peroxidase-like activity and were assembled with an aptamer composed of a thiol-modified epithelial specific cell adhesion molecule (EpCAM) to strengthen CTCs adhesion. Moreover, the electrode surface was decorated with highly fractal Au nanostructures (HFAuNSs) composites due to the similarity in fractal nanostructure with the CTCs membrane to enhance the CTCs anchoring efficiency and release capability. The captured CTCs could be further efficiently dissociated and nondestructively released from the modified electrodes upon electrochemical reductive desorption. The designed cytosensor showed an excellent analytical performance, with a wide linear range from 2 to 1 × 105 cells mL−1 and low limit of detection (LOD) of 2 cells mL−1 (S/N = 3) at the working potential in the range −0.6 to 0.2 V. A satisfactory CTCs release reaching a range of 93.7–97.4% with acceptable RSD from 3.55 to 6.41% and good cell viability was obtained. Thus, the developed cytosensor might provide a potential alternative to perform CTC-based liquid biopsies, with promising applications in early diagnosis of tumors.
Graphical abstract

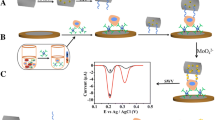
Preparation and mechanism of desorption of the cytosensor based on 2D Au@PdMo nanozymes and electrochemical reductive desorption for the detection and release of CTCs. A Preparation procedure of the Apt/Au@PbMo bioconjugates. B Fabrication process of the sandwich-type cytosensor. C Electrochemical signal produced by the Au@PdMo nanozymes. D Mechanism of electrochemical reductive desorption for CTCs release





Similar content being viewed by others
References
Jackson JM, Witek MA, Kamande JW, Soper SA (2017) Materials and microfluidics: enabling the efficient isolation and analysis of circulating tumour cells. Chem Soc Rev 46(14):4245–4280. https://doi.org/10.1039/C7CS00016B
Kilgour E, Rothwell DG, Brady G, Dive C (2020) Liquid biopsy-based biomarkers of treatment response and resistance. Cancer Cell 37(4):485–495. https://doi.org/10.1016/j.ccell.2020.03.012
Pantel K, Alix-Panabieres C (2019) Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol 16(7):409–424. https://doi.org/10.1038/s41571-019-0187-3
Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S, Ke Z, Tseng HR (2014) Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res 47:2941–2950. https://doi.org/10.1021/ar5001617
Parkinson D, Dracopoli N, Petty B, Compton C, Cristofanilli M, Deisseroth A, Hayes D, Kapke G, Kumar P, Lee J, Liu M, McCormack R, Mikulski S, Nagahara L, Pantel K, Pearson-White S, Punnoose E, Roadcap L, Schade A, Scher H, Sigman C, Kelloff G (2012) Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 10:138. https://doi.org/10.1186/1479-5876-10-138
Chemi F, Rothwell DG, McGranahan N, Gulati S, Abbosh C, Pearce SP, Zhou C, Wilson GA, Jamal-Hanjani M, Birkbak N, Pierce J, Kim CS, Ferdous S, Burt DJ, Slane-Tan D, Gomes F, Moore D, Shah R, Al Bakir M, Hiley C, Veeriah S, Summers Y, Crosbie P, Ward S, Mesquita B, Dynowski M, Biswas D, Tugwood J, Blackhall F, Miller C, Hackshaw A, Brady G, Swanton C, Dive C, Consortium TR (2019) Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med 25(10):1534–1539. https://doi.org/10.1038/s41591-019-0593-1
Teutsch SM, Bradley LA, Palomaki GE, Haddow JE, Piper M, Calonge N, Dotson WD, Douglas MP, Berg AO, Group EW (2009) The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: methods of the EGAPP working group. Genet Med 11(1):3–14. https://doi.org/10.1097/GIM.0b013e318184137c
Zhou X, Li Y, Wu H, Huang W, Ju H, Ding S (2019) A amperometric immunosensor for sensitive detection of circulating tumor cells using a tyramide signal amplification-based signal enhancement system. Biosens Bioelectron 130:88–94. https://doi.org/10.1016/j.bios.2019.01.023
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 48(4):1004–1076. https://doi.org/10.1039/c8cs00457a
Wei H, Wang EK (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42:6060–6093. https://doi.org/10.1039/c3cs35486e
Campuzano S, Pedrero M, Yáñez-Sedeño P, Pingarrón JJ (2020) Nanozymes in electrochemical affinity biosensing. Mikrochim Acta 187:423. https://doi.org/10.1007/s00604-020-04390-9
Liang M, Wang Y, Ma K, Yu S, Chen Y, Deng Z, Liu Y, Wang F (2020) Engineering inorganic nanoflares with elaborate enzymatic specificity and efficiency for versatile biofilm eradication. Small 16:e2002348. https://doi.org/10.1002/smll.202002348
Li X, Li X, Li D, Zhao M, Wu H, Shen B, Liu P, Ding SJ (2020) Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@Pt@MOF nanozyme. Biosens Bioelectron 168:112554. https://doi.org/10.1016/j.bios.2020.112554
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan XY (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. https://doi.org/10.1038/nnano.2007.260
Liu W, Guo J, Chen C, Ni P, Jiang Y, Zhang C, Wang B, Lu YZ (2021) Ultrathin PdCu alloy nanosheet-assembled 3D nanoflowers with high peroxidase-like activity toward colorimetric glucose detection. Mikrochim Acta 188:114. https://doi.org/10.1007/s00604-021-04776-3
Liu X, Yan Z, Zhang Y, Liu Z, Sun Y, Ren J, Qu X (2019) Two-dimensional metal-organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano 13(5):5222–5230. https://doi.org/10.1021/acsnano.8b09501
Mu J, He L, Fan W, Tang W, Wang Z, Jiang C, Zhang D, Liu Y, Deng H, Zou J, Jacobson O, Qu J, Huang P, Chen X (2020) Cascade reactions catalyzed by planar metal-organic framework hybrid architecture for combined cancer therapy. Small 16(42):e2004016. https://doi.org/10.1002/smll.202004016
You J, Liu L, Huang W, Manners I, Dou HJ (2021) In situ redox-active micelle-based reaction platforms for preparation of noble metal nanocomposites with photothermal conversion capability. ACS Appl Mater Interfaces 13:13648–13657. https://doi.org/10.1021/acsami.0c21925
Zhang H, Liang F, Wu X, Liu Y, Chen A (2020) Recognition and sensitive detection of CTCs using a controllable label-free electrochemical cytosensor. Mikrochim Acta 187:487. https://doi.org/10.1007/s00604-020-04452-y
Wang H, Wan K, Shi X (2019) Recent advances in nanozyme research. Adv Mater 31:e1805368. https://doi.org/10.1002/adma.201805368
Feng L, Zhang L, Zhang S, Chen X, Li P, Gao Y, Xie S, Zhang A, Wang H (2020) Plasma-assisted controllable doping of nitrogen into MoS nanosheets as efficient nanozymes with enhanced peroxidase-like catalysis activity. ACS Appl Mater Interfaces 12:17547–17556. https://doi.org/10.1021/acsami.0c01789
Zhou X, Luo B, Kang K, Zhang Y, Jiang P, Lan F, Yi Q, Wu Y (2019) Leukocyte-repelling biomimetic immunomagnetic nanoplatform for high-performance circulating tumor cells isolation. Small 15(17):e1900558. https://doi.org/10.1002/smll.201900558
Yan S, Chen P, Zeng X, Zhang X, Li Y, Xia Y, Wang J, Dai X, Feng X, Du W, Liu BF (2017) Integrated multifunctional electrochemistry microchip for highly efficient capture, release, lysis, and analysis of circulating tumor cells. Anal Chem 89:12039–12044. https://doi.org/10.1021/acs.analchem.7b02469
Dong J, Chen JF, Smalley M, Zhao M, Ke Z, Zhu Y, Tseng HR (2020) Nanostructured substrates for detection and characterization of circulating rare cells: from materials research to clinical applications. Adv Mater 32:e1903663. https://doi.org/10.1002/adma.201903663
Shen H, Deng W, He Y, Li X, Song J, Liu R, Liu H, Yang G, Li L (2020) Ultrasensitive aptasensor for isolation and detection of circulating tumor cells based on CeO2@Ir nanorods and DNA walker. Biosens Bioelectron 168:112516. https://doi.org/10.1016/j.bios.2020.112516
Zhu L, Feng X, Yang S, Wang J, Pan Y, Ding J, Li C, Yin X, Yu Y (2021) Colorimetric detection of immunomagnetically captured rare number CTCs using mDNA-wrapped single-walled carbon nanotubes. Biosens Bioelectron 172:112780. https://doi.org/10.1016/j.bios.2020.112780
Zhang M, Zhai Q, Wan L, Chen L, Peng Y, Deng C, Xiang J, Yan JW (2018) Electrochemical impedance spectroscopy for real-time detection of lipid membrane damage based on a porous self-assembly monolayer support. Anal Chem 90:7422–7427. https://doi.org/10.1021/acs.analchem.8b00884
Dokukin M, Guz N, Gaikwad R, Woodworth C, Sokolov I (2011) Cell surface as a fractal: normal and cancerous cervical cells demonstrate different fractal behavior of surface adhesion maps at the nanoscale. Phys Rev Lett 107:028101. https://doi.org/10.1103/PhysRevLett.107.028101
Zhang P, Chen L, Xu T, Liu H, Liu X, Meng J, Yang G, Jiang L, Wang S (2013) Programmable fractal nanostructured interfaces for specific recognition and electrochemical release of cancer cells. Adv Mater 25:3566–3570. https://doi.org/10.1002/adma.201300888
Luo M, Zhao Z, Zhang Y, Sun Y, Xing Y, Lv F, Yang Y, Zhang X, Hwang S, Qin Y, Ma JY, Lin F, Su D, Lu G, Guo S (2019) PdMo bimetallene for oxygen reduction catalysis. Nature 574(7776):81–85. https://doi.org/10.1038/s41586-019-1603-7
Chen J, Zhang J, Yang H, Fu F, Chen G (2010) A strategy for development of electrochemical DNA biosensor based on site-specific DNA cleavage of restriction endonuclease. Biosens Bioelectron 26:144–148. https://doi.org/10.1016/j.bios.2010.05.033
Han D, Yan Y, Wang J, Zhao M, Duan X, Kong L, Wu H, Cheng W, Min X, Ding S (2019) An enzyme-free electrochemiluminesce aptasensor for the rapid detection of Staphylococcus aureus by the quenching effect of MoS2-PtNPs-vancomycin to S2O82−/O2 system. Sensors Actuators B Chem 288:586–593. https://doi.org/10.1016/j.snb.2019.03.050
Pan D, Fang Z, Yang E, Ning Z, Zhou Q, Chen K, Zheng Y, Zhang Y, Shen YF (2020) Facile preparation of WO3-x dots with remarkably low toxicity and uncompromised activity as co-reactants for clinical diagnosis by electrochemiluminescence. Angew Chem Int Ed Eng 59(38):16747–16754. https://doi.org/10.1002/anie.202007451
Cao Y, Zhu W, Wei H, Ma C, Lin Y, Zhu JJ (2020) Stable and monochromatic all-inorganic halide perovskite assisted by hollow carbon nitride nanosphere for ratiometric electrochemiluminescence bioanalysis. Anal Chem 92(5):4123–4130. https://doi.org/10.1021/acs.analchem.0c00070
Gu C, Guo C, Li Z, Wang M, Zhou N, He L, Zhang Z, Du M (2019) Bimetallic ZrHf-based metal-organic framework embedded with carbon dots: ultra-sensitive platform for early diagnosis of HER2 and HER2-overexpressed living cancer cells. Biosens Bioelectron 134:8–15. https://doi.org/10.1016/j.bios.2019.03.043
Hashemi P, Afkhami A, Baradaran B, Halabian R, Madrakian T, Arduini F, Nguyen T, Bagheri H (2020) Well-orientation strategy for direct immobilization of antibodies: development of the immunosensor using the boronic acid-modified magnetic graphene nanoribbons for ultrasensitive detection of lymphoma cancer cells. Anal Chem 92(16):11405–11412. https://doi.org/10.1021/acs.analchem.0c02357
Wang H, Zhou C, Sun X, Jian Y, Kong Q, Cui K, Ge S, Yu JH (2018) Polyhedral-AuPd nanoparticles-based dual-mode cytosensor with turn on enable signal for highly sensitive cell evalution on lab-on-paper device. Biosens Bioelectron 117:651–658. https://doi.org/10.1016/j.bios.2018.07.004
Peng Y, Pan Y, Han Y, Sun Z, Jalalah M, Al-Assiri M, Harraz F, Yang J, Li G (2020) Direct analysis of rare circulating tumor cells in whole blood based on their controlled capture and release on electrode surface. Anal Chem 92(19):13478–13484. https://doi.org/10.1021/acs.analchem.0c02906
Peng Y, Peng Y, Tang S, Shen H, Sheng S, Wang Y, Wang T, Cai J, Xie G, Feng W (2020) PdIrBP mesoporous nanospheres combined with superconductive carbon black for the electrochemical determination and collection of circulating tumor cells. Mikrochim Acta 187(4):216. https://doi.org/10.1007/s00604-020-4213-z
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP (2017) The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99. https://doi.org/10.3322/caac.21388
Funding
This research was funded by the financial support from the National Science and Technology Major Project of the Ministry of Science and Technology of China (2018ZX10732202), the National Natural Science Foundation of China (81873980), the Natural Science Foundation Project of Chongqing (cstc2018jcyjAX0349), and the Zhejiang Provincial Natural Science Fund (LQ21H200005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 4896 kb)
Rights and permissions
About this article
Cite this article
Yang, W., Fan, L., Guo, Z. et al. Reversible capturing and voltammetric determination of circulating tumor cells using two-dimensional nanozyme based on PdMo decorated with gold nanoparticles and aptamer. Microchim Acta 188, 319 (2021). https://doi.org/10.1007/s00604-021-04927-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04927-6