Abstract
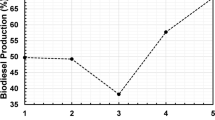
Catalytic activity of materials prepared by means of sodium ion-exchange by potassium and cesium in NaY zeolite, was studied in the deoxygenation of methyl laurate, as biodiesel model compound. The increase of the Ion-exchange produced a basicity increase that leads to a lower deactivation in the deoxygenation of methyl laurate, reaching a steady conversion of about 92% for 4 h (time on stream, TOS) with the CsKY zeolite. However, the potassium ion exchange leaded to higher production of bio-hydrocarbons, especially unsaturated (96% of the C10, C11 and C12 production), with higher production of C11 by means of decarbonylation. Furthermore, mesoporosity on the KY zeolite was generated, using SDBS (sodium dodecylbenzene sulphonate) during the synthesis, with the aim of increasing its capacity to process bulky molecules. The presence of mesoporosity in the potassium zeolite allowed a higher tolerance to deactivation maintaining a steady conversion of about 90% for 4 h TOS. The yield to desirable products (C10 to C12 hydrocarbons) was about 37%. Therefore, biofuel upgrading can be carried out using potassium Y zeolite with mesoporosity.







Similar content being viewed by others
References
S. van Donk, A.H. Janssen, J.H. Bitter, K.P. de Jong, Generation, Characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. (2003). https://doi.org/10.1081/CR-120023908
J. Čejka, S. Mintova, Perspectives of micro/mesoporous composites in catalysis. Catal. Rev. (2007). https://doi.org/10.1080/01614940701583240
J. Prech, P. Pizarro, D.P. Serrano, J. Cejka, From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. (2018). https://doi.org/10.1039/C8CS00370J
A. Feliczak-Guzik, Hierarchical zeolites: synthesis and catalytic properties. Microporous Mesoporous Mater. (2018). https://doi.org/10.1016/j.micromeso.2017.09.030
T. Pan, Z. Wu, A.C.K. Yip, Advances in the green synthesis of microporous and hierarchical zeolites: a short review. Catalysts (2019). https://doi.org/10.3390/catal9030274
D. Verboekend, N. Nuttens, R. Locus, J. van Aelst, P. Verolme, J.C. Groen, J. Pérez-Ramirez, B.F. Sels, Synthesis, characterization, and catalytic evaluation of hierarchical faujasite zeolites: milestones, challenges, and future directions. Chem. Soc. Rev. (2016). https://doi.org/10.1039/C5CS00520E
J.M. Gómez, E. Díez, A. Rodríguez, M. Calvo, Synthesis of mesoporous X zeolite using an anionic surfactant as templating agent for thermo-catalytic deoxygenation. Microporous Mesoporous Mater. (2018). https://doi.org/10.1016/j.micromeso.2018.05.029
M.A. Peralta, T. Sooknoi, T. Danuthai, D.E. Resasco, Deoxygenation of benzaldehyde over CsNaX zeolites. J. Mol. Catal. A: Chem. (2009). https://doi.org/10.1016/j.molcata.2009.07.008
T. Sooknoi, T. Danuthai, L.L. Lobban, R.G. Mallinson, D.E. Resasco, Deoxygenation of methylesters over CsNaX. J. Catal. (2008). https://doi.org/10.1016/j.jcat.2008.06.012
J.M. Gómez, M.D. Romero, V. Callejo, Heterogeneous basic catalysis for upgrading of biofuels. Catal. Today (2013). https://doi.org/10.1016/j.cattod.2013.04.027
J.M. Gómez, E. Díez, A. Rodríguez, R. Palanca, Thermocatalytic deoxygenation of methyl laurate over potassium FAU zeolites. Microporous Mesoporous Mater. (2019). https://doi.org/10.1016/j.micromeso.2019.04.025
M.-Y. Choo, J.C. Juan, L.E. Oi, T.C. Ling, E.P. Ng, A.R. Noorsaadah, G. Centi, K.T. Lee, The role of nanosized zeolite Y in the H2-free catalytic deoxygenation of triolein. Catal. Sci. Technol. (2019). https://doi.org/10.1039/C8CY01877D
H. Hernando, B. Puértolas, P. Pizarro, J. Fermoso, J. Pérez-Ramírez D.P. Serrano. Cascade Deoxygenation Process Integrating Acid and Base Catalysts for the Efficient Production of Second-Generation Biofuels. ACS Sustainable Chem. Eng. (2019) https://doi.org/https://doi.org/10.1021/acssuschemeng.9b04921
R. Cerný, M. Kubu, D. Kubicka The effect of oxygenates structure on their deoxygenation over USY zeolite Catalysis Today (2013) https://doi.org/10.1016/j.cattod.2012.07.041
Z. Pan, R. Wang, J. Chena, Catalytic deoxygenation of methyllaurate as a model compound to hydrocarbons on hybrid catalysts composed of Ni–Zn alloy and HYzeolite. J. Chem. Technol. Biotechnol. (2019). https://doi.org/10.1002/jctb.5943
J.M. Crawford , C.S. Smoljan , J.L., M.A. Carreon. Deoxygenation of Stearic Acid over Cobalt-Based NaX Zeolite Catalysts. Catalysts (2019). https://doi.org/10.3390/catal9010042
D. W. Breck. Zeolite Molecular Sieves. John Wiley & Sons, New York, NY, USA, 1974. https://doi.org/10.1093/chromsci/13.4.18A-c
M.D. Romero, A. Rodríguez, J.M. Gómez, Basicity in Zeolites, in New Topics in Catalysis Research. ed. by D.K. McReynolds (Nova Science Publishers Inc, New York, 2006), pp. 197–220
W.J. Mortier, Compilation of Extra Framework Sites in Zeolites (Butterworth-Heinemann, London, 1982).
D.H. Olson, The crystal structure of dehydrated NaX. Zeolites (1995). https://doi.org/10.1016/0144-2449(95)00029-6
J.C. Groen, L.A.A. Peffer, J. Pérez-Ramírez. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. (2003). https://doi.org/10.1016/S1387-1811(03)00339-1
M.E. Davis, P.E. Hathaway, Base catalysis by alkali modified zeolites: III Alkylation with methanol. J. Catal. (1989). https://doi.org/10.1016/0021-9517(89)90177-2
Acknowledgements
This work was supported by the financial support of the Santander-UCM 2018 project (PR75718).
Author information
Authors and Affiliations
Contributions
JMG: Conceptualization, Methodology, Writing-Original draft preparation, Supervision, ED: Methodology, Writing-Original draft preparation, AR: Writing—Review and Editing, Supervision, CJ: Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gómez, J.M., Díez, E., Rodríguez, A. et al. Deoxygenation of methyl laurate: influence of cation and mesoporosity in fau zeolites. J Porous Mater 28, 1355–1360 (2021). https://doi.org/10.1007/s10934-021-01086-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01086-0