Abstract
Purpose
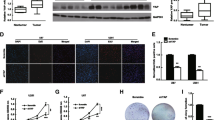
Gliomas are the most common and aggressive malignant brain tumors and are associated with high mortality and incidence in humans. Despite rigorous multi-modal therapy, including surgery, chemotherapy and radiotherapy, patients with malignant glioma survive an average of 12–15 months following primary diagnosis. Therefore, new molecular biomarkers are urgently needed for diagnosis and targeted therapy. Here, we find that suppression of CKAP4 might inhibit glioma growth through regulation of Hippo signaling.
Methods
We examined the expression levels of CKAP4 through analysis of RNA sequencing data from GEPIA and CGGA databases. Then, Lentivirus was used to construct stable cell lines with knockout or overexpression of CKAP4. Next, the function of CKAP4 on glioma was investigated in vitro and in an orthotopic brain tumor model in mice. Lastly, luciferase reporter assay, immunofluorescence and immunoblotting were performed to explore the potential mechanism of how CKAP4 affects gliomas.
Results
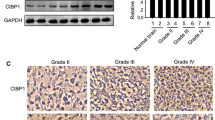
CKAP4 is highly upregulated in glioma and high CKAP4 expressing tumors were associated with poor patient survival. And CKAP4 promotes malignant progression of gliomas via inhibiting Hippo signaling.
Conclusion
CKAP4 has potential as a promising biomarker and can predict the prognosis of patients with gliomas. And targeting CKAP4 expression may be an effective therapeutic strategy for the treatment of human gliomas.





Similar content being viewed by others
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the risk of compromising individual privacy but are available from the corresponding author on reasonable request and provided that an appropriate collaboration agreement can be agreed upon.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA 67:7–30. https://doi.org/10.3322/caac.21387
McNeill KA (2016) Epidemiology of brain tumors. Neurol Clin 34:981–998. https://doi.org/10.1016/j.ncl.2016.06.014
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 19:1–88. https://doi.org/10.1093/neuonc/nox158
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2020) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol 22:1–96. https://doi.org/10.1093/neuonc/noaa200
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA 60:166–193. https://doi.org/10.3322/caac.20069
Tang Y, Fang G, Guo F, Zhang H, Chen X, An L, Chen M, Zhou L, Wang W, Ye T, Zhou L, Nie P, Yu H, Lin M, Zhao Y, Lin X, Yuan Z, Jiao S, Zhou Z (2020) Selective inhibition of STRN3-containing PP2A phosphatase restores hippo tumor-suppressor activity in gastric cancer. Cancer Cell 38(115–128):e119. https://doi.org/10.1016/j.ccell.2020.05.019
Yu FX, Zhao B, Guan KL (2015) Hippo pathway in organ size control, tissue omeostasis, and cancer. Cell 163:811–828. https://doi.org/10.1016/j.cell.2015.10.044
Zhang H, Geng D, Gao J, Qi Y, Shi Y, Wang Y, Jiang Y, Zhang Y, Fu J, Dong Y, Gao S, Yu R, Zhou X (2016) Expression and significance of Hippo/YAP signaling in glioma progression. Tumour Biol. https://doi.org/10.1007/s13277-016-5318-1
Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, Jiang X (2019) Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572:402–406. https://doi.org/10.1038/s41586-019-1426-6
Zeng Q, Hong W (2008) The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13:188–192. https://doi.org/10.1016/j.ccr.2008.02.011
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21:2747–2761. https://doi.org/10.1101/gad.1602907
Zhu C, Li L, Zhao B (2015) The regulation and function of YAP transcription co-activator. Acta Biochim Biophys Sin 47:16–28. https://doi.org/10.1093/abbs/gmu110
McNeill H, Woodgett JR (2010) When pathways collide: collaboration and connivance among signalling proteins in development. Nat Rev Mol Cell Biol 11:404–413. https://doi.org/10.1038/nrm2902
Ji J, Ding K, Luo T, Xu R, Zhang X, Huang B, Chen A, Zhang D, Miletic H, Bjerkvig R, Thorsen F, Wang J, Li X (2020) PMEPA1 isoform a drives progression of glioblastoma by promoting protein degradation of the Hippo pathway kinase LATS1. Oncogene 39:1125–1139. https://doi.org/10.1038/s41388-019-1050-9
Ding K, Ji J, Zhang X, Huang B, Chen A, Zhang D, Li X, Wang X, Wang J (2019) RNA splicing factor USP39 promotes glioma progression by inducing TAZ mRNA maturation. Oncogene 38:6414–6428. https://doi.org/10.1038/s41388-019-0888-1
Ji J, Xu R, Zhang X, Han M, Xu Y, Wei Y, Ding K, Wang S, Bin H, Chen A, Di Z, Jiang Z, Xu S, Zhang Q, Li W, Ni S, Wang J, Li X (2018) Actin like-6A promotes glioma progression through stabilization of transcriptional regulators YAP/TAZ. Cell Death Dis 9:517. https://doi.org/10.1038/s41419-018-0548-3
Gargini R, Escoll M, Garcia E, Garcia-Escudero R, Wandosell F, Anton IM (2016) WIP drives tumor progression through YAP/TAZ-dependent autonomous cell growth. Cell Rep 17:1962–1977. https://doi.org/10.1016/j.celrep.2016.10.064
Rivas S, Anton IM, Wandosell F (2018) WIP-YAP/TAZ as a new pro-oncogenic pathway in glioma. Cancers. https://doi.org/10.3390/cancers10060191
Sun S, Irvine KD (2016) Cellular organization and cytoskeletal regulation of the hippo signaling network. Trends Cell Biol 26:694–704. https://doi.org/10.1016/j.tcb.2016.05.003
Zhang J, Zhou Y, Tang PMK, Cheng ASL, Yu J, To KF, Kang W (2019) Mechanotransduction and cytoskeleton remodeling shaping YAP1 in gastric tumorigenesis. Int J Mol Sci. https://doi.org/10.3390/ijms20071576
Vedrenne C, Hauri H-P (2006) Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic 7:639–646. https://doi.org/10.1111/j.1600-0854.2006.00419.x
Razzaq TM, Bass R, Vines DJ, Werner F, Whawell SA, Ellis V (2003) Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type-II transmembrane protein p63 (CKAP4). J Biol Chem 278:42679–42685. https://doi.org/10.1074/jbc.M305695200
Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK (2006) CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem 281:37836–37843. https://doi.org/10.1074/jbc.M604581200
Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H, Eguchi H, Shintani Y, Endo H, Inoue M, Doki Y, Okumura M, Morii E, Kikuchi A (2016) CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Investig 126:2689–2705. https://doi.org/10.1172/JCI84658
Bhavanasi D, Speer KF, Klein PS (2016) CKAP4 is identified as a receptor for Dickkopf in cancer cells. J Clin Investig 126:2419–2421. https://doi.org/10.1172/JCI88620
Li SX, Tang GS, Zhou DX, Pan YF, Tan YX, Zhang J, Zhang B, Ding ZW, Liu LJ, Jiang TY, Hu HP, Dong LW, Wang HY (2014) Prognostic significance of cytoskeleton-associated membrane protein 4 and its palmitoyl acyltransferase DHHC2 in hepatocellular carcinoma. Cancer 120:1520–1531. https://doi.org/10.1002/cncr.28593
Kimura H, Yamamoto H, Harada T, Fumoto K, Osugi Y, Sada R, Maehara N, Hikita H, Mori S, Eguchi H, Ikawa M, Takehara T, Kikuchi A (2019) CKAP4, a DKK1 receptor, is a biomarker in exosomes derived from pancreatic cancer and a molecular target for therapy. Clin Cancer Res 25:1936–1947. https://doi.org/10.1158/1078-0432.CCR-18-2124
Kajiwara C, Fumoto K, Kimura H, Nojima S, Asano K, Odagiri K, Yamasaki M, Hikita H, Takehara T, Doki Y, Morii E, Kikuchi A (2018) p63-Dependent Dickkopf3 expression promotes esophageal cancer cell proliferation via CKAP4. Can Res 78:6107–6120. https://doi.org/10.1158/0008-5472.CAN-18-1749
Gao L, Wang Q, Ren W, Zheng J, Li S, Dou Z, Kong X, Liang X, Zhi K (2020) The RBP1-CKAP4 axis activates oncogenic autophagy and promotes cancer progression in oral squamous cell carcinoma. Cell Death Dis 11:488. https://doi.org/10.1038/s41419-020-2693-8
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45:W98–W102. https://doi.org/10.1093/nar/gkx247
Calses PC, Crawford JJ, Lill JR, Dey A (2019) Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends Cancer 5:297–307. https://doi.org/10.1016/j.trecan.2019.04.001
Zanconato F, Cordenonsi M, Piccolo S (2016) YAP/TAZ at the roots of cancer. Cancer Cell 29:783–803. https://doi.org/10.1016/j.ccell.2016.05.005
Harvey KF, Zhang X, Thomas DM (2013) The Hippo pathway and human cancer. Nat Rev Cancer 13:246–257. https://doi.org/10.1038/nrc3458
Funding
This work was supported by the National Natural Science Foundation of China (81903126 and 81972351), the Special Foundation for Taishan Scholars (ts20110184, tshw201502056, tsqn201909173 and tsqn20161067), the Department of Science & Technology of Shandong Province (ZR2019ZD33), the China Postdoctoral Science Foundation (2018M642666 and 2020T130371), the Jinan Science and Technology Bureau of Shandong Province (2019GXRC006), the 111 Project (B20058), the Shandong research institute of industrial technology.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. TL, JW, and XL conceived and designed the project. TL, KD, JJ, XZ, XY performed experiments. TL, KD, JJ analyzed the data. TL, KD, JJ wrote the manuscript. AC, BH, DZ provided reagents and materials. JW, and XL supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
All co-authors declare no potential conflicts of interest.
Ethics approval and consent to participate
The protocols in the present study involving human samples were approved by the Research Ethics Committee of Shandong University (Shandong, China), and the study was conducted in accordance with the Declaration of Helsinki. The animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Shandong University and performed under the guidance of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All participants in the study provided written informed consent.
Consent for publication
Written informed consent for publication of their clinical details was obtained from the relative of the patient. A copy of the consent form is available for review by the Editor of this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luo, T., Ding, K., Ji, J. et al. Cytoskeleton-associated protein 4 (CKAP4) promotes malignant progression of human gliomas through inhibition of the Hippo signaling pathway. J Neurooncol 154, 275–283 (2021). https://doi.org/10.1007/s11060-021-03831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03831-6