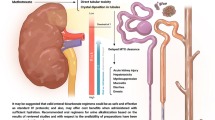
Abstract
Purpose
Intravenous (IV) sodium bicarbonate is considered standard therapy for high-dose methotrexate (HDMTX) urine alkalinization. Due to a national IV sodium bicarbonate shortage, an oral (PO) sodium bicarbonate protocol was implemented by Alberta Health Services (AHS) for HDMTX urine alkalinization. This study aims to evaluate the efficacy and safety of the PO sodium bicarbonate protocol compared to IV sodium bicarbonate for HDMTX urine alkalinization.
Methods
A retrospective chart review of adult patients who received HDMTX (> 500 mg/m2) with sodium bicarbonate for urine alkalinization at 4 hospitals in Alberta was conducted. Patients who received IV sodium bicarbonate between January and June 2017 and PO sodium bicarbonate between July and December 2017 were compared for the primary outcome of time to methotrexate clearance.
Results
A total of 84 and 78 HDMTX cycles were included in the IV and PO cohorts, respectively. No difference in time to methotrexate clearance was seen between the IV and PO cohorts, 91.6 (± 35.4) hours and 95.2 (± 44) hours respectively; p = 0.5. The proportion of HDMTX cycles that experienced a > 25% increase in serum creatinine was not statistically significant, IV protocol 12% and PO protocol 5%; p = 0.13. Nausea and emesis occurred more frequently in the PO cohort than the IV cohort, though rarely resulted in refused doses or change to alternate sodium bicarbonate formulations.
Conclusions
The results of this study indicate that the AHS PO sodium bicarbonate protocol was no different in time to methotrexate clearance or rates of increased serum creatinine when compared to IV sodium bicarbonate.

Similar content being viewed by others
References
Howard SC, McCormick J, Pui C et al (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21:1471–1482
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11:694–703
Drost SA, Wentzell JR, Giguere P et al (2017) Outcomes associated with reducing the urine alkalinization threshold in patients receiving high-dose methotrexate. Pharmacotherapy 37(6):684–691
Alrabiah Z, Luter D, Proctor A, Bates JS (2015) Substitution of sodium acetate for sodium bicarbonate for urine alkalinization in high-dose methotrexate therapy. Am J Health-Syst Pharm 72(15):1932–1934
Sterrett SP, Penniston KL, Wolf JS et al (2008) Acetazolamide is an effective adjunct for urinary alkalinization in patients with uric acid and cystine stone formation recalcitrant to potassium citrate. Urology 72:278–281
Shamash J, Earl H, Souhami R (1991) Acetazolamide for alkalinization of urine in patients receiving high-dose methotrexate. Cancer Chemother Pharmacol 25(2):150–151
Whiteside H, Gandhi A, Ajebo G, Bryan LJ (2018) When baking soda goes on shortage: urine alkalinization with acetazolamide and oral sodium bicarbonate. Ann Pharmacother 52(3):297–298
Cohen B, Laish I, Brosh-Nissimov T, Hoffman A, Katz LH, Braunstein R, Sagi R, Michael G (2013) Efficacy of urine alkalinization by oral administration of sodium bicarbonate: a prospective open-label trial. Am J Emerg Med 31:1703–1706
Roy AM, Lei M, Lou U (2019) Safety and efficacy of a urine alkalinization protocol developed for high-dose methotrexate patients during intravenous bicarbonate shortage. J Oncol Pharm Practice 25(8):1860–1866
Kramer E, Filtz M, Pace M (2020) Evaluation of methotrexate clearance with an enteral urine alkalinization protocol for patients receiving high-dose methotrexate. J Oncol Pharm Practice. 27:26–32. https://doi.org/10.1177/1078155220908946
Rouch JA, Burton B, Dabb A, Brown V, Seung AH, Kinsman K, Holdhoff M (2017) Comparison of enteral and parenteral methods of urine alkalinization in patients receiving high-dose methotrexate. J Oncol Pharm Practice 23(1):3–9
Diachinsky M, Tran T, Jupp J, McKinnon K (2020) Oral sodium bicarbonate protocol for high-dose methotrexate urine alkalinization: a pediatric experience. J Oncol Pharm Practice 27:119–127. https://doi.org/10.1177/1078155220915769
Visage R, Kaiser N, Williams M, Kim A (2019) Oral method of urinary alkalinization for high-dose methotrexate administration: alternatives to intravenous sodium bicarbonate during a critical drug shortage. J Pediatr Hematol Oncol 41(5):371–375
Reed DR, Pierce EJ, Sen JM, Keng MK (2019) A prospective study on urine alkalinization with an oral regimen consisting of sodium bicarbonate and acetazolamide in patients receiving high-dose methotrexate. Cancer Manag Res 11:8065–8072
Schmickl CN, Owen RL et al (2020) Side effects of acetazolamide: a systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Resp Res 7:e000557. https://doi.org/10.1136/bmjresp-2020-000557
Swenson ER (2014) Safety of carbonic anhydrase inhibitors. Expert Opin Drug Saf 13(4):459–472
Maisey DN, Brown RD (1981) Acetazolamide and symptomatic metaboic acidosis in mild renal failure. Br Med J (Clin Res Ed) 283(6305):1527–1528
Pampin R, Lebeaga Y, Rodriguez B et al (2019) Experience with ambulatory high-dose methotrexate administration as CNS prophylaxis in patients with non-Hodgkin lymphoma. J Oncol Pharm Practice 26(3):549–555
Bernard S, Hachon L, Diasonama J et al (2021) Ambulatory high-dose methotrexate administration as central nervous system prophylaxis in patients with aggressive lymphoma. Ann Hematol 100:979–986. https://doi.org/10.1007/s00277-020-04341-7
Acknowledgements
The authors would like to thank Matthew Newman, PharmD, BCOP, and The Johns Hopkins Hospital Department of Pharmacy, Weinberg Oncology Division, for their assistance with the study. We would also like to thank Melanie Varghese, BSc Pharm; Tyler Moore, BSc Pharm; Deonne Dersch-Mills, BSc Pharm, ACPR, PharmD; Ashley Yim; Eric Duong; Alexandra Yuriyivna Spirkina; and Jessica Kim for their contributions.
Availability of data and material
The authors maintain control of all the data used for the completion of this study. Anonymized data can be made available upon request.
Code availability
N/A
Funding
No funding was received for the completion of this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: Jennifer C. Jupp. Methodology: Jennifer C. Jupp, Jennifer L. Hawker, Leanne G. Fong, Josee Z. Rioux, Janet L. Quon, and Jordan J. Kelly. Project administration and supervision: Jennifer C. Jupp. Data collection: Jordan J. Kelly. Data analysis: Rachel D. Heisler. Writing—original draft: Rachel D. Heisler and Sara Abedinzadegan Abdi. Writing—review and editing: Rachel D. Heisler, Sara Abedinzadegan Abdi, Jennifer C. Jupp, Jennifer L. Hawker, Leanne G. Fong, Josee Z. Rioux, and Janet L. Quon.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from The Health Research Ethics Board of Alberta Cancer Committee (HREBA-CC).
Consent to participate
A waiver of consent to participate was obtained.
Consent to publish
A waiver of consent to publish was obtained.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Johns Hopkins Hospital Oral Sodium Bicarbonate Protocol
Sodium Bicarbonate Shortage – Changes in Urine Alkalinization for IV High-Dose Methotrexate (≥ 5 g/m2) | ||
|---|---|---|
Recommendations (assuming an 80 kg patient) | Total daily sodium bicarbonate | |
Patients who are able to tolerate PO or have alternate enteral access (i.e., PEG tube) | Pre-admission: Sodium bicarbonate 1300 mg PO q6 hours OR Bicitra 20 ml PO q8 hours starting one day prior to planned methotrexate administration. Upon admission: If urine pH ≥ 7.5: D5½NS IV at 3 ml/kg/h, and continue pre-methotrexate urine alkalinization regimen. If urine pH < 7.5: • Start D5½NS IV at 3 ml/kg/h, and bolus with Bicitra 120 mL orally/enterally × 1 dose. • Continue D5½NS IV at 3 ml/kg/h, and give sodium bicarbonate 9750 mg (15 tablets) orally/enterally q6 hours OR Bicitra 120 mL orally/enterally q6 hours. Bolus with an additional oral/enteral dose of Bicitra 120 mL if urine pH < 7.5 | Pre-admission: 64 mEq (tablets); 60 mEq (Bicitra) During admission: 480 mEq |
Patients unable to tolerate PO, but have enteral access (i.e., PEG tube) | Pre-admission: Bicitra 20 ml enterally q8 hours starting one day prior to planned methotrexate administration. Upon admission: If urine pH ≥ 7.5: D5½ NS IV at 3 ml/kg/h, and continue pre-methotrexate urine alkalinization regimen. If urine pH < 7.5: •Start D5½ NS IV at 3 ml/kg/h and bolus with Bicitra 120 mL enterally × 1 dose. • Continue D5½NS IV at 3 ml/kg/h, and give Bicitra 120 mL enterally q6 hours. Bolus with additional enteral dose of Bicitra 120 mL if urine pH < 7.5. | Pre-admission: 60 mEq (Bicitra) During admission: 480 mEq |
Patients with no enteral access | • Sodium bicarbonate 100 mEq/L IV at 3 mL/kg/h OR • Start D5½NS IV at 3 ml/kg/h, and place NG tube, and administer Bicitra 120 ml via NG tube q6 hours. Bolus with an additional NG dose of Bicitra 120 mL if urine pH < 7.5. | 576 mEq/day 480 mEq/day |
Sodium Bicarbonate Shortage-Changes in Urine Alkalinization for IV Intermidiate/Moderate-Dose Methotraxate (> 1 g/m2 and < 5 g/m2) | ||
|---|---|---|
Recommendations (assuming an 80 kg patient) | Total daily sodium bicarbonate | |
Patients who are able to tolerate PO or have alternate enteral access (i.e., PEG tube) | Pre-admission: Sodium bicarbonate 1300 mg PO q6 hours OR Bicitra 20 ml PO q8 hours starting one day prior to planned methotrexate administration. Upon admission: If urine pH ≥ 7: Lactated Ringer’s IV at ml/kg/h, and continue pre-methotrexate urine alkalinization regimen. If urine pH < 7: • Start Lactated Ringer’s IV at 2 ml/kg/h and bolus with Bicitra 120 ml orally/enterally × 1 dose. • Continue Lactated Ringer’s IV at 2 ml/kg/h and give sodium bicarbonate 9750 mg (15 tablets) orally/enterally q6 hours OR Bicitra 120 mL orally/enterally q6 hours. Bolus with an additional oral/enteral dose of Bicitra 120 mL if urine pH < 7. | Pre-admission: 64 mEq (tablets); 60 mEq (Bicitra) During admission: 588 mEq |
Patients unable to tolerate PO, but have enteral access (i.e., PEG tube) | Pre-admission: Bicitra 20 ml enterally q8 hours starting one day prior to planned methotrexate administration. Upon admission: If urine pH ≥ 7: Lactated Ringer’s IV at 2 ml/kg/h, and continue pre-methotrexate urine alkalinization regimen. If urine pH < 7: •Start Lactated Ringer’s IV at 2 ml/kg/h and bolus with Bicitra 120 mL enterally × 1 dose. •Continue Lactated Ringer’s IV at 2 ml/kg/h, and give Bicitra 120 mL enterally q6 hours. Bolus with additional enteral dose of Bicitra 120 mL if urine pH < 7. | Pre-admission: 60 mEq (Bicitra) During admission: 588 mEq |
Patients unable to tolerate PO, but have enteral access (i,e., PEG tube) | Pre-admission: Bicitra 20 ml enterally q8 hours starting one day prior to planned methotrexate admission. Upon admission: If urine pH ≥ 7: Lactated Ringer’s IV at 2 ml/kg/h and continue pre-methotrexate urine alkalinization regimen. If urine pH < 7: • Start Lactated Ringer’s IV at 2 ml/kg/h and bolus with Bicitra 120 mL enterally × 1 dose. • Continue Lactated Ringer’s IV at 2 ml/kg/h and give Bicitra 120 mL enterally q6 hours. Bolus with additional enteral dose of Bicitra 120 mL if urine pH < 7. | Pre-admission: 60 mEq (Bicitra During admission: 588 mEq |
Patients with no enteral access | • Sodium bicarbonate 100 mEq/L IV at 2 mL/kg/h OR • Start Lactated Ringer’s IV at 2 mL/kg/h, place NG tube, and administer Bicitra 120 ml via NG tube q6 hours. Bolus with additional NG dose of Bicitra 120 mL if urine pH < 7. | 576 mEq 588 mEq |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heisler, R.D., Kelly, J.J., Abedinzadegan Abdi, S. et al. Evaluation of an oral sodium bicarbonate protocol for high-dose methotrexate urine alkalinization. Support Care Cancer 30, 1273–1281 (2022). https://doi.org/10.1007/s00520-021-06503-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06503-3