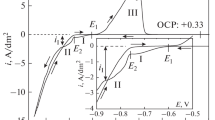
Abstract
Factors leading to size-dependent surface properties of electroplated coatings obtained by induced codeposition of iron group metals and tungsten are investigated. The size effect in microhardness described in earlier studies and the size effect in the corrosion resistance revealed in this work are shown to stem from a common origin, which is the formation of surface oxides. Removing surface oxides by abrasive processing leads to a higher corrosion rate and cancels the size effect in microhardness. Factors contributing to the formation of surface oxide layers during induced codeposition of considered alloys are studied.










Similar content being viewed by others
REFERENCES
Eliaz, N. and Gileadi, N., Induced codeposition of alloys of tungsten, molybdenum and rhenium with transition metals, in Modern Aspects of Electrochemistry, New York: Springer-Verlag, 2008, vol. 42, p. 191.
Tsyntsaru, N., Cesiulis, H., Donten, M., Sort, J., et al., Modern trends in tungsten alloys electrodeposition with iron group metals, Surf. Eng. Appl. Electrochem., 2012, vol. 48, no. 6, p. 491.
Cesiulis, H., Tsyntsaru, N., Podlaha, E., Li, D., et al., Electrodeposition of iron-group alloys in to nanostructured oxide membranes: synthetic challenges and properties, Curr. Nanosci., 2018, vol. 14, p. 1.
Brenner, A., Electrodeposition of Alloys: Principle and Practice, New York: Academic, 1963.
Silkin, S.A., Gotelyak, A.V., Tsyntsaru, N., Dikusar, A.I., Size effect of microhardness of nanocrystalline Co–W coatings produced from citrate and gluconate solutions, Surf. Eng. Appl. Electrochem., 2015, vol. 51, no. 3, p. 228.
Belevskii, S.S., Bobanova, Zh.I., Buravets V.A, Gotekyak, V.A., et al., Electrodeposition of Co–W coatings from boron gluconate electrolyte with a soluble tungsten anode, Russ. J. Appl. Chem., 2016, vol. 89, no. 4, p. 1427.
Gotelyak, A.V., Silkin, S.A., Yahova, E.A., Dikusar, A.I., Effect of pH and volume current density on deposition rate and microhardness of Co–W coatings electrodeposited from concentrated boron-gluconate electrolyte, Russ. J. Appl. Chem., 2017, vol. 90, no. 4, p. 541.
Silkin, S.A., Gotelyak, A.V., Tsyntsaru, N., Dikusar, A.I., Electrodeposition of alloys of the iron-group metals with tungsten from citrate and gluconate solutions: size effect of microhardness, Surf. Eng. Appl. Electrochem., 2017, vol. 53, no. 1, p. 6.
Danilchuk, V.V., Silkin, S.A., Gotelyak, A.V., Buravets V.A., et al., The microhardness properties and rate of electrodeposition of Co–W alloys from boro-gluconate bath: Impact of anodic processes, Russ. J. Electrochem., 2018, vol. 54, no. 11, p. 930.
Belevskii, S.S., Gotelyak, A.V., Silkin, S.A., and Dikusar, A.I., Macroscopic size effect on the microhardness of electroplated iron group metal–tungsten alloy coatings: Impact of electrode potential and oxygen-containing impurities, Surf. Eng. Appl. Electrochem., 2019, vol. 55, no. 1, p. 46.
Belevskii, S.S., Danilchuk, V.V., Gotelyak, A.V., Lelis, M., et al., Electrodeposition of Fe–W alloys from citrate bath: Impact of anode material, Surf. Eng. Appl. Electrochem., 2020, vol. 56, no. 1, p. 1.
Myrzak, V.A., Globa, P.G., Sidelinikova, S.P., and Dikusar, A.I., The size effect of the corrosion rate of a copper nanowire array. Part I: The corrosion potential variation, Surf. Eng. Appl. Electrochem., 2012, vol. 48, no. 5, p. 412.
Myrzak, V.A. and Dikusar, A.I., On size effect of rate of corrosion of copper nanowire ensemble: Part 2. Size effect of rate of corrosion of copper in pyrophosphate solution, Surf. Eng. Appl. Electrochem., 2016, vol. 52, no. 2, p. 140.
Podlaha, E.J. and Landolt, D., Induced codeposition: I. An experimental investigation of Ni–Mo alloys, J. Electrochem. Soc., 1996, vol. 143, p. 889.
Podlaha, E.J. and Landolt, D., Induced codeposition: II. Mathematical modeling of Ni–Mo alloys, J. Electrochem. Soc., 1996, vol. 143, p. 893.
Krasikov, V.L., The role of electrochemical cobalt reduction intermediates in formation of oxygen-containing admixtures, Izv. S.-Peterb. Gos. Tekh. Univ., 2015, no. 31, p. 40. https://doi.org/10.15217/issn1998984-9.2015.31.40
Krasikov, V.L. and Krasikov, A.V., Electrodeposition mechanism of nickel-tungsten alloy from pyrophosphate electrolyte, Izv. S.-Peterb. Gos. Tekh. Univ., 2016, no. 36, pp. 12–23. https://doi.org/10.15217/issn1998984-9.2016.36.12
Krasikov, A.V. and Krasikov, V.L., Mechanism for induced codeposition of alloys and some single refractory metals, Izv. S.-Peterb. Gos. Tekh. Univ., 2016, no. 37, pp. 8–14. https://doi.org/10.15217/issn1998984-9.2016.37.8
Bobanova, Zh., Petrenko, V., Tsyntsaru, N., and Dikusar, A., Leveling power of Co–W and Fe–W electrodeposited coatings, Key. Eng. Mater., 2019, vol. 813, p. 248.
Belevskii, S.S., Cesiulis, H., Tsynysaru, N., and Dikusar, A.I., The role of mass transfer in the formation of the composition and structure of Co–W coatings electrodeposited from citrate solutions, Surf. Eng. Appl. Electrochem., 2010, vol. 46, no. 6, p. 570.
Gamburg, Yu.D. and Zakharov, E.N., Electrodeposition of ternary Fe–W–H alloys, Surf. Eng. Appl. Electrochem., 2019, vol. 55, no. 4, p. 402.
Petrii, O.A. and Tsirlina, G.A., Size effects in electrochemistry, Russ. Chem. Rev., 2001, vol. 70, no. 4, p. 285.
Funding
This work was supported by the National Agency for Research and Development, Republic of Moldova, within the project “Manufacturing of New Micro- and Nanostructuring Materials by Physicochemical Methods and the Elaboration on their Base” (no. 19.80013.50.07.06 A/BL) and in part by the Europe-funded project H2020 Smartelectrodes (no. 778357) and budget funding of the Shevchenko Pridnestrovie State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Kukharuk
About this article
Cite this article
Myrzak, V., Gotelyak, A.V. & Dikusar, A.I. Size Effects in the Surface Properties of Electroplated Alloys between Iron Group Metals and Tungsten. Surf. Engin. Appl.Electrochem. 57, 409–418 (2021). https://doi.org/10.3103/S1068375521040128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375521040128