Abstract
Background
Huntington’s disease (HD) is a neurodegenerative disorder characterized by involuntary movements, cognitive decline, and behavioral changes. The complex constellation of clinical symptoms still makes the therapeutic management challenging. In the new era of functional neurosurgery, deep brain stimulation (DBS) may represent a promising therapeutic approach in selected HD patients.
Methods
Articles describing the effect of DBS in patients affected by HD were selected from Medline and PubMed by the association of text words with MeSH terms as follows: “Deep brain stimulation,” “DBS,” and “HD,” “Huntington’s disease,” and “Huntington.” Details on repeat expansion, age at operation, target of operation, duration of follow-up, stimulation parameters, adverse events, and outcome measures were collected.
Results
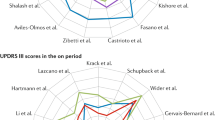
Twenty eligible studies, assessing 42 patients with HD, were identified. The effect of globus pallidus internus (GPi) DBS on Unified Huntington’s Disease Rating Scale (UHDRS) total score revealed in 10 studies an improvement of total score from 5.4 to 34.5%, and in 4 studies, an increase of motor score from 3.8 to 97.8%. Bilateral GPi-DBS was reported to be effective in reducing Chorea subscore in all studies, with a mean percentage reduction from 21.4 to 73.6%.
Conclusions
HD patients with predominant choreic symptoms may be the best candidates for surgery, but the role of other clinical features and of disease progression should be elucidated. For this reason, there is a need for more reliable criteria that may guide the selection of HD patients suitable for DBS. Accordingly, further studies including functional outcomes as primary endpoints are needed.


Similar content being viewed by others
References
Caron NS, Wright GEB, Hayden MR (1998) Huntington disease. [updated 2020 Jun 11]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews® [Internet]. University of Washington, Seattle, pp 1993–2020
Bonomo R, Latorre A, Balint B, Smilowska K, Rocchi L, Rothwell JC, Zappia M, Bhatia KP (2020) Voluntary inhibitory control of chorea: a case series. Mov Disord Clin Pract 7(3):308–312. https://doi.org/10.1002/mdc3.12907
Kowall NM, Ferrante RJ, Martin JB (1987) Patterns of cell loss in Huntington’s disease. Trends Neurosci 10:24–29
Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB (1988) Differential loss of striatal projection neurons in Huntington’s disease. Proc Natl Acad Sci USA 64:397–404
Albin RL, Young AB, Penney JB, Handelin B, Balfour KD, Markel DS, Tourtelotte WW, Reiner A (1990) Abnormalities of striatal projection neurons and N-methyl-d-aspartate receptors in presymptomatic Huntington’s disease. New Engl J Med 322:1293–1298
Sapp E, Ge P, Aizawa H, Bird E, Penney J, Young AB, Vonsattel JP, DiFiglia M (1995) Evidence for a preferential loss of enkephalin immunoreactivity in the external globus pallidus in low grade Huntington’s disease using high resolution image analysis. Neuroscience 64:397–404
Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS (2008) Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Exp Neurol 211(1):227–233
Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV (2008) Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington’s disease. J Neurophysiol 100(4):2205–2216
Miller BR, Walker AG, Fowler SC, von Horsten S, Riess O, Johnson MA, Rebec GV (2010) Dysregulation of coordinated neuronal firing patterns in striatum of freely behaving transgenic rats that model Huntington’s disease. Neurobiol Dis 37(1):106–113
Penney JB, Young AB (1986) Striatal inhomogeneities and basal ganglia function. Mov Disord 1:3–15
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Berardelli A, Noth J, Thompson PD et al (1999) Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord 14(3):398–403
Joel D, Ayalon L, Tarrasch R, Veenman L, Feldon J, Weiner I (1998) Electrolytic lesion of globus pallidus ameliorates the behavioural and neurodegenerative effects of quinolinic acid lesion of the striatum: a potential novel treatment in a rat model of Huntington’s disease. Brain Res 787:143–148
Spiegel EA, Wycis HT (1952) Thalamotomy and pallidotomy for treatment of choreic movements. Acta neuroch 2:417–422. https://doi.org/10.1007/BF01405833
De Vloo P, Breen DP, Milosevic L, Lee DJ, Dallapiazza RF, Hutchison WD, Lang AE, Lozano AM (2019) Successful pallidotomy for post-hyperglycemic hemichorea-ballism. Parkinsonism Relat Disord 61:228–230. https://doi.org/10.1016/j.parkreldis.2018.11.023
Watarai M, Hashimoto T, Yamamoto K, Matsumoto Y, Tada T, Ikeda S (2003) Pallidotomy for severe generalized chorea of juvenile-onset dentatorubral-pallidoluysian atrophy. Neurology 61(10):1452–1454. https://doi.org/10.1212/01.wnl.0000094202.26313.73
Fujimoto Y, Isozaki E, Yokochi F, Yamakawa K, Takahashi H, Hirai S (1997) A case of chorea-acanthocytosis successfully treated with posteroventral pallidotomy. Rinsho Shinkeigaku 37(10):891–894 (Japanese)
Edwards TC, Zrinzo L, Limousin P, Foltynie T (2012) Deep brain stimulation in the treatment of chorea. Mov Disord 27(3):357–363. https://doi.org/10.1002/mds.23967
Vitek JL, Jones R, Bakay REA, Hersch SM (2000) Pallidotomy for Huntington’s disease. Ann Neurol 48:429
Wichmann T, DeLong MR (2016) Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics 13(2):264–283. https://doi.org/10.1007/s13311-016-0426-6
Florence G, Sameshima K, Fonoff ET, Hamani C (2016) Deep brain stimulation: more complex than the inhibition of cells and excitation of fibers. Neuroscientist 22(4):332–345. https://doi.org/10.1177/1073858415591964
Lozano AM, Lipsman N, Bergman H et al (2019) Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15(3):148–160. https://doi.org/10.1038/s41582-018-0128-2
Ferrea S, Groiss SJ, Elben S, Hartmann CJ, Dunnett SB, Rosser A, Saft C, Schnitzler A, Vesper J, Wojtecki L (2018) Surgical approaches working group of the European Huntington’s disease network (EHDN). Pallidal deep brain stimulation in juvenile Huntington’s disease: local field potential oscillations and clinical data. J Neurol 265(7):1573–1579. https://doi.org/10.1007/s00415-018-8880-1
Zittel S, Moll CK, Gulberti A, Tadic V, Rasche D, Bäumer T, Fellbrich A, Brüggemann N, Engel AK, Tronnier V, Hamel W, Münchau A (2015) Pallidal deep brain stimulation in Huntington’s disease. Parkinsonism Relat Disord 21(9):1105–1108. https://doi.org/10.1016/j.parkreldis.2015.06.018
Zittel S, Tadic V, Moll CKE, Bäumer T, Fellbrich A, Gulberti A, Rasche D, Brüggemann N, Tronnier V, Münchau A (2018) Prospective evaluation of Globus pallidus internus deep brain stimulation in Huntington’s disease. Parkinsonism Relat Disord 51:96–100. https://doi.org/10.1016/j.parkreldis.2018.02.030
Delorme C, Rogers A, Lau B, Francisque H, Welter ML, Vidal SF, Yelnik J, Durr A, Grabli D, Karachi C (2016) Deep brain stimulation of the internal pallidum in Huntington’s disease patients: clinical outcome and neuronal firing patterns. J Neurol 263(2):290–298. https://doi.org/10.1007/s00415-015-7968-0
Gonzalez V, Cif L, Biolsi B, Garcia-Ptacek S, Seychelles A, Sanrey E, Descours I, Coubes C, de Moura AM, Corlobe A, James S, Roujeau T, Coubes P (2014) Deep brain stimulation for Huntington’s disease: long-term results of a prospective open-label study. J Neurosurg 121(1):114–122. https://doi.org/10.3171/2014.2.JNS131722
Wojtecki L, Groiss SJ, Ferrea S, Elben S, Hartmann CJ, Dunnett SB, Rosser A, Saft C, Südmeyer M, Ohmann C, Schnitzler A, Vesper J (2015) Surgical approaches working group of the European Huntington’s disease network (EHDN). A prospective pilot trial for pallidal deep brain stimulation in Huntington’s disease. Front Neurol 18(6):177. https://doi.org/10.3389/fneur.2015.00177
Beste C, Mückschel M, Elben S, Hartmann J, McIntyre CC, Saft C, Vesper J, Schnitzler A, Wojtecki L (2015) Behavioral and neurophysiological evidence for the enhancement of cognitive control under dorsal pallidal deep brain stimulation in Huntington’s disease. Brain Struct Funct 220(4):2441–2448. https://doi.org/10.1007/s00429-014-0805-x
Velez-Lago FM, Thompson A, Oyama G, Hardwick A, Sporrer JM, Zeilman P, Foote KD, Bowers D, Ward HE, Sanchez-Ramos J, Okun MS (2013) Differential and better response to deep brain stimulation of chorea compared to dystonia in Huntington’s disease. Stereotact Funct Neurosurg 91(2):129–133. https://doi.org/10.1159/000341070
Kang GA, Heath S, Rothlind J, Starr PA (2011) Long-term follow-up of pallidal deep brain stimulation in two cases of Huntington’s disease. J Neurol Neurosurg Psychiatry 82(3):272–277. https://doi.org/10.1136/jnnp.2009.202903
Gruber D, Kuhn AA, Schoenecker T, Kopp UA, Kivi A, Huebl J, Lobsien E, Mueller B, Schneider GH, Kupsch A (2014) Quadruple deep brain stimulation in Huntington’s disease, targeting pallidum and subthalamic nucleus: case report and review of the literature. J Neural Transm (Vienna) 121(10):1303–1312. https://doi.org/10.1007/s00702-014-1201-7
Spielberger S, Hotter A, Wolf E, Eisner W, Müller J, Poewe W, Seppi K (2012) Deep brain stimulation in Huntington’s disease: a 4-year follow-up case report. Mov Disord 27(6):806–807; author reply 807–8. doi: https://doi.org/10.1002/mds.24959
Vedam-Mai V, Martinez-Ramirez D, Hilliard JD, Carbunaru S, Yachnis AT, Bloom J, Keeling P, Awe L, Foote KD, Okun M (2016) Post-mortem findings in Huntington’s deep brain stimulation: a moving target due to atrophy. Tremor Other Hyperkinet Mov (N Y) 6:372
Biolsi B, Cif L, Fertit HE, Robles SG, Coubes P (2008) Long-term follow-up of Huntington disease treated by bilateral deep brain stimulation of the internal globus pallidus. J Neurosurg 109(1):130–132. https://doi.org/10.3171/JNS/2008/109/7/0130
Cislaghi G, Capiluppi E, Saleh C, Romano L, Servello D, Mariani C, Porto M (2014) Bilateral globus pallidus stimulation in Westphal variant of Huntington disease. Neuromodulation 17(5):502–505. https://doi.org/10.1111/ner.12098
Fasano A, Mazzone P, Piano C, Quaranta D, Soleti F, Bentivoglio AR (2008) GPi-DBS in Huntington’s disease: results on motor function and cognition in a 72-year-old case. Mov Disord 23(9):1289–1292. https://doi.org/10.1002/mds.22116
Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, Hutchison WD, Lozano AM (2004) Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol 56(2):290–294. https://doi.org/10.1002/ana.20183
Fawcett AP, Moro E, Lang AE, Lozano AM, Hutchison WD (2005) Pallidal deep brain stimulation influences both reflexive and voluntary saccades in Huntington’s disease. Mov Disord 20(3):371–377. https://doi.org/10.1002/mds.20356 (PMID: 15580556)
López-Sendón Moreno JL, García-Caldentey J, Regidor I, del Álamo M, García de Yébenes J (2014) A 5-year follow-up of deep brain stimulation in Huntington’s disease. Parkinsonism Relat Disord 20(2):260–261. https://doi.org/10.1016/j.parkreldis.2013.11.007
Huys D, Bartsch C, Poppe P, Lenartz D, Huff W, Prütting J, Timmermann L, Klosterkötter J, Maarouf M, Rommel T, Hartmann A, Sturm V, Kuhn J (2013) Management and outcome of pallidal deep brain stimulation in severe Huntington’s disease. Fortschr Neurol Psychiatr 81(4):202–205. https://doi.org/10.1055/s-0033-1335097
Hebb MO, Garcia R, Gaudet P, Mendez IM (2006) Bilateral stimulation of the globus pallidus internus to treat choreathetosis in Huntington’s disease: technical case report. Neurosurgery 58(2):E383; discussion E383. https://doi.org/10.1227/01.NEU.0000195068.19801.18
Loutfi G, Linder J, Hariz G, Hariz M, Blomstedt P (2014) Pallidal deep brain stimulation in the treatment of Huntington’s chorea. Brain Disord Therapy 3(4):2. https://doi.org/10.4172/2168-975X.1000136
Yin Z, Bai Y, Zhang H, Liu H, Hu W, Meng F, Yang A, Zhang J (2020) An individual patient analysis of the efficacy of using GPi-DBS to treat Huntington’s disease. Brain Stimul 13(6):1722–1731. https://doi.org/10.1016/j.brs.2020.09.025
Ravina B, Romer M, Constantinescu R, Biglan K, Brocht A, Kieburtz K, Shoulson I, McDermott MP (2008) The relationship between CAG repeat length and clinical progression in Huntington’s disease. Mov Disord 23(9):1223–1227. https://doi.org/10.1002/mds.21988
Mahant N, McCusker EA, Byth K, Graham S, Huntington Study Group (2003) Huntington’s disease: clinical correlates of disability and progression. Neurology 61(8):1085–1092. https://doi.org/10.1212/01.wnl.0000086373.32347.16 (PMID: 14581669)
Ross CA, Pantelyat A, Kogan J, Brandt J (2014) Determinants of functional disability in Huntington’s disease: role of cognitive and motor dysfunction. Mov Disord 29(11):1351–1358. https://doi.org/10.1002/mds.26012
Frank S (2009) Tetrabenazine as anti-chorea therapy in Huntington disease: an open-label continuation study. Huntington Study Group/TETRA-HD Investigators. BMC Neurol 9:62. https://doi.org/10.1186/1471-2377-9-62
Ligot N, Krystkowiak P, Simonin C et al (2011) External globus pallidus stimulation modulates brain connectivity in Huntington’s disease. J Cereb Blood Flow Metab 31(1):41–46. https://doi.org/10.1038/jcbfm.2010.186
Aziz NA, Jurgens CK, Landwehrmeyer GB, van Roon-Mom WMC, van Ommen JGB, Stijnen T, Roos RAC et al (2009) Normal and mutant HTT interact to affect clinical severity and progression in Huntington disease. Neurology 73:1280–1285
Chao TK, Hu J, Pringsheim T (2017) Risk factors for the onset and progression of Huntington disease. Neurotoxicology 61:79–99
Grahn PJ, Mallory GW, Khurram OU et al (2014) A neurochemical closed-loop controller for deep brain stimulation: toward individualized smart neuromodulation therapies. Front Neurosci 8:169. https://doi.org/10.3389/fnins.2014.00169
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonomo, R., Elia, A.E., Bonomo, G. et al. Deep brain stimulation in Huntington’s disease: a literature review. Neurol Sci 42, 4447–4457 (2021). https://doi.org/10.1007/s10072-021-05527-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05527-1