Abstract
Purpose
Cancer care team attitudes towards distress screening are key to its success and sustainability. Previous qualitative research has interviewed staff mostly around the startup phase. We evaluate oncology teams’ perspectives on psychosocial distress screening, including perceived strengths and challenges, in settings where it has been operational for years.
Methods
We conducted, transcribed, and analyzed semi-structured interviews with 71 cancer care team members (e.g., MDs, RNs, MSWs) at 18 Commission on Cancer-accredited cancer programs including those serving underrepresented populations.
Results
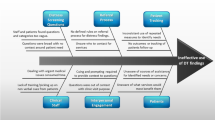
Strengths of distress screening identified by participants included identifying patient needs and testing provider assumptions. Staff indicated it improved patient-provider communication and other aspects of care. Challenges to distress screening included patient barriers (e.g., respondent burden) and lack of electronic system interoperability. Participants expressed the strengths of distress screening (n = 291) more than challenges (n = 86). Suggested improvements included use of technology to collect data, report results, and make referrals; complete screenings prior to appointments; longitudinal assessment; additional staff training; and improve resources to address patient needs.
Conclusion
Cancer care team members’ perspectives on well-established distress screening programs largely replicate findings of previous studies focusing on the startup phase, but there are important differences: team members expressed more strengths than challenges, suggesting a positive attitude. While our sample described many challenges described previously, they did not indicate challenges with scoring and interpreting the distress screening questionnaire. The differences in attitudes expressed in response to mature versus startup implementations provide important insights to inform efforts to sustain and optimize distress screening.

Similar content being viewed by others
Data availability
Research data are not shared.
Code availability
NVivo 12 qualitative data analysis software was used.
References
NCCN Clinical Practice Guidelines in Oncology: Distress Management (version 2). National Comprehensive Cancer Network
American Cancer Society (2019) Cancer treatment & survivorship facts & figures 2019–2021. American Cancer Society
Carlson LE, Waller A, Mitchell AJ (2012) Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol 30:1160–1177. https://doi.org/10.1200/JCO.2011.39.5509
Cimino T, Said K, Safier L et al (2020) Psychosocial distress among oncology patients in the safety net. Psychooncology. https://doi.org/10.1002/pon.5525
(2016) Cancer Program Standards: Ensuring Patient-Centered Care. American College of Surgeons Commission on Cancer
Mallin K, Browner A, Palis B et al (2019) Incident cases captured in the national cancer database compared with those in U.S. population based central cancer registries in 2012–2014. Ann Surg Oncol 26:1604–1612. https://doi.org/10.1245/s10434-019-07213-1
Zhang B, Lloyd W, Jahanzeb M, Hassett MJ (2018) Use of patient-reported outcome measures in quality oncology practice initiative-registered practices: results of a national survey. J Oncol Pract 14:e602–e611. https://doi.org/10.1200/JOP.18.00088
Smith TG, Bontemps-Jones J, James TA, McCabe RM Feasibility and utility of abstracting data from policy and procedure documents to describe real world distress screening: a case study of 18 U.S. Cancer Centers. Submitted
Anatchkova M, Donelson SM, Skalicky AM et al (2018) Exploring the implementation of patient-reported outcome measures in cancer care: need for more real-world evidence results in the peer reviewed literature. J Patient Rep Outcomes 2:64. https://doi.org/10.1186/s41687-018-0091-0
Howell D, Molloy S, Wilkinson K et al (2015) Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol 26:1846–1858. https://doi.org/10.1093/annonc/mdv181
Knies AK, Jutagir DR, Ercolano E et al (2019) Barriers and facilitators to implementing the commission on cancer’s distress screening program standard. Palliat Support Care 17:253–261. https://doi.org/10.1017/S1478951518000378
NicGiollaEaspaig B, Tran Y, Bierbaum M et al (2020) What are the attitudes of health professionals regarding patient reported outcome measures (PROMs) in oncology practice? A mixed-method synthesis of the qualitative evidence. BMC Health Serv Res 20:102. https://doi.org/10.1186/s12913-020-4939-7
Boyce MB, Browne JP, Greenhalgh J (2014) The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf 23:508–518. https://doi.org/10.1136/bmjqs-2013-002524
Chambers DA, Glasgow RE, Stange KC (2013) The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci 8:117. https://doi.org/10.1186/1748-5908-8-117
Yin RK (2002) Case study research: design and methods, 3rd edn. SAGE Publications Inc, Thousand Oaks
Glaser BG, Strauss AL (2017) Discovery of grounded theory: strategies for qualitative research. Routledge
Schilling J (2006) On the pragmatics of qualitative assessment: designing the process for content analysis. Eur J Psychol Assess 22:28–37. https://doi.org/10.1027/1015-5759.22.1.28
Lincoln YS, Guba EG Naturalistic Inquiry. SAGE Publications, Newbury Park
(2018) NVivo qualitative data analysis software. QSR International Pty Ltd
Miller NS, McCabe RM, Knutson A (2016) The Commission on Cancer Psychosocial Distress Screening Standard: the first year in review. JCO 34:198–198. https://doi.org/10.1200/jco.2016.34.3_suppl.198
Piderit SK (2000) Rethinking resistance and recognizing ambivalence: a multidimensional view of attitudes toward an organizational change. AMR 25:783–794. https://doi.org/10.5465/amr.2000.3707722
Basch E, Deal AM, Kris MG et al (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34:557–565. https://doi.org/10.1200/JCO.2015.63.0830
Chen J, Ou L, Hollis SJ (2013) A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res 13:211. https://doi.org/10.1186/1472-6963-13-211
Atkinson TM, Ryan SJ, Bennett AV et al (2016) The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 24:3669–3676. https://doi.org/10.1007/s00520-016-3297-9
Newell S, Sanson-Fisher RW, Girgis A, Bonaventura A (1998) How well do medical oncologists’ perceptions reflect their patients’ reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer 83:1640–1651
NCCN Guidelines for Supportive Care. In: National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed 10 Jul 2020
Managing Symptoms, Side Effects & Well-Being. In: Cancer Care Ontario. https://www.cancercareontario.ca/en/symptom-management. Accessed 3 Jul 2020
Jensen RE, Snyder CF, Abernethy AP et al (2014) Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract 10:e215-222. https://doi.org/10.1200/JOP.2013.001067
Jensen RE, Rothrock NE, DeWitt EM et al (2015) The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care 53:153–159. https://doi.org/10.1097/MLR.0000000000000289
Johansen MA, Henriksen E, Horsch A et al (2012) Electronic symptom reporting between patient and provider for improved health care service quality: a systematic review of randomized controlled trials. part 1: state of the art. J Med Internet Res 14:e118. https://doi.org/10.2196/jmir.2214
Vasileiou K, Barnett J, Thorpe S, Young T (2018) Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med Res Methodol 18. https://doi.org/10.1186/s12874-018-0594-7
Marshall B, Cardon P, Poddar A, Fontenot R (2015) Does sample size matter in qualitative research?: a review of qualitative interviews in is research. J Comput Inform Syst 54:11–22. https://doi.org/10.1080/08874417.2013.11645667
Acknowledgements
We appreciate cancer center staff who participated in this study. The content of this publication does not necessarily reflect the views of the American Cancer Society.
Funding
This study is intramurally funded by the American Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The Sterling Institutional Review Board determines this study (IRBID: 6308) as exempt from IRB review because participant interview responses gathered during this study would not “reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation” in the unlikely event they were accidentally disclosed outside of research.
Consent to participate
A consent form was emailed to participants and verbal informed consent was obtained prior to the interview.
Consent for publication
The consent form and verbal consent included consent to publish data captured during the interviews.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, T.G., Beckwitt, A.E., van de Poll-Franse, L.V. et al. Oncology team perspectives on distress screening: a multisite study of a well-established use of patient-reported outcomes for clinical assessment. Support Care Cancer 30, 1261–1271 (2022). https://doi.org/10.1007/s00520-021-06458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06458-5