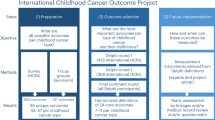
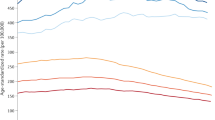
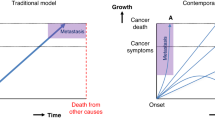
Abstract
Cancer represents an important cause of disease-related death in children worldwide. Improved treatment and understanding of the ways in which cancer manifests has allowed for a greater prospect of survival in children of all ages. However, variation in childhood cancer experience exists based on factors at the individual, community and systems levels. Throughout the cancer care continuum these factors may influence the access and timeliness of care a child receives, leading to delays in diagnosis and treatment. The pejorative designation ‘delay in diagnosis and treatment’ is better characterised as lag time, representing an interval that is thought to influence survival and overall outcome. In recent decades, work has been done to expedite early childhood cancer diagnosis through the creation of screening and education-based programmes. Although systematic cancer screening in children poses risks and fails to achieve the goal of early diagnosis, a case has been made for risk-based surveillance that has been shown to improve outcome and reduce occurrence of advanced stage disease in targeted populations. The components of lag time are examined separately and individually. This review highlights the challenges of early diagnosis in childhood cancers and describes important contributors in the cancer care continuum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the GLOBOCAN and SEER data repositories at https://gco.iarc.fr/today and https://seer.cancer.gov/csr/1975_2018/
Change history
16 September 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41416-021-01548-x
References
Steliarova‐Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer. 2005;103:1457–67.
Steliarova-Foucher E, Colombet M, Ries LA, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18:719–31.
Stewart BW, Wild CP. World Cancer Report 2014. Lyon: The International Agency for Research on Cancer; 2014.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48.
Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20:483–93.
Rodriguez-Galindo C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. 2015;33:3065.
De Camargo B, De Andrea MLM, Franco EL. Catching up with history: treatment of Wilms’ tumor in a developing country. Med Pediatr Oncol. 1987;15:270–6.
Bhakta N, Force LM, Allemani C, Atun R, Bray F, Coleman MP, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20:e42–e53.
Gupta S, Howard SC, Hunger SP, Antillon FG, Metzger ML, Israels T, et al. Treating Childhood Cancer in Low- and Middle-Income Countries. In: H Gelband, P Jha, R Sankaranarayanan, S Horton, editors. Cancer: Disease Control Priorities, 3rd edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development/The World Bank; 2015 Nov 1. Chapter 7. 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343626/.
Abramson DH, Beaverson K, Sangani P, Vora RA, Lee TC, Hochberg HM, et al. Screening for retinoblastoma: presenting signs as prognosticators of patient and ocular survival. Pediatrics. 2003;112:1248–55.
Erwenne CM, Franco EL. Age and lateness of referral as determinants of extra-ocular retinoblastoma. Ophthalmic Paediatr Genet. 1989;10:179–84.
Neal R, Tharmanathan P, France B, Din N, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92–S107.
Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–85.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today. Accessed 21 July 2021.
Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2018. Bethesda: National Cancer Institute. https://seer.cancer.gov/csr/1975_2018/. Accessed 21 July 2021.
Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012;379:1436–46.
Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429.
Force LM, Abdollahpour I, Advani SM, Agius D, Ahmadian E, Alahdab F, et al. The global burden of childhood and adolescent cancer in 2017: an analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019;20:1211–25.
Benson JR, Jatoi I, Keisch M, Esteva FJ, Makris A, Jordan VC, et al. Early breast cancer. Lancet. 2009;373:1463–79.
Franco EL, Duarte-Franco E, Rohan TE. Evidence-based policy recommendations on cancer screening and prevention. Cancer Detect Prev. 2002;26:350–61.
Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95.
Pollock BH, Krischer JP, Vietti TJ. Interval between symptom onset and diagnosis of pediatric solid tumors. J Pediatr. 1991;119:725–32.
Stiller CA. Epidemiology and genetics of childhood cancer. Oncogene. 2004;23:6429–44.
Wilson JMG, Jungner G. Principles and practice of screening for disease. In: Public health papers; no. 34. Geneva: World Health Organization; 1968.
Tota JE, Isidean SD, Franco EL. Defining benchmarks for tolerable risk thresholds in cancer screening: Impact of HPV vaccination on the future of cervical cancer screening. Int J Cancer. 2020;147:3305–12.
Woods WG, Tuchman M, Robison LL, Bernstein M, Leclerc J-M, Brisson LC, et al. A population-based study of the usefulness of screening for neuroblastoma. Lancet. 1996;348:1682–7.
Sawada T, Nakata T, Takasugi N, Maeda K, Hanawa Y, Shimizu K, et al. Mass screening for neuroblastoma in infants in Japan: interim report of a mass screening study group. Lancet. 1984;324:271–3.
Schilling FH, Spix C, Berthold F, Erttmann R, Fehse N, Hero B, et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346:1047–53.
Soderstrom L, Woods WG, Bernstein M, Robison LL, Tuchman M, Lemieux B, et al. Health and economic benefits of well-designed evaluations: some lessons from evaluating neuroblastoma screening. J Natl Cancer Inst. 2005;97:1118–24.
Woods WG, Gao R-N, Shuster JJ, Robison LL, Bernstein M, Weitzman S, et al. Screening of infants and mortality due to neuroblastoma. N Engl J Med. 2002;346:1041–6.
Schilling FH, Spix C, Berthold F, Erttmann R, Sander J, Treuner J, et al. Children may not benefit from neuroblastoma screening at 1 year of age. Updated results of the population based controlled trial in Germany. Cancer Lett. 2003;197:19–28.
Yamamoto K, Hanada R, Kikuchi A, Ichikawa M, Aihara T, Oguma E, et al. Spontaneous regression of localized neuroblastoma detected by mass screening. J Clin Oncol. 1998;16:1265–9.
Brodeur G, Look A, Shimada H, Hamilton V, Maris J, Hann H, et al. Biological aspects of neuroblastomas identified by mass screening in Quebec. Med Pediatr Oncol. 2001;36:157–9.
Barrette S, Bernstein ML, Leclerc J-M, Champagne MA, Samson Y, Brossard J, et al. Treatment complications in children diagnosed with neuroblastoma during a screening program. J Clin Oncol. 2006;24:1542–5.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13.
Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–7.
Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2016;27:316.
Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S, et al. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25:1127–36.
Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract. 2015;21:686–96.
Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma. Nat Rev Dis Prim. 2015;1:1–23.
Committee on Practice Ambulatory Medicine. Eye examination in infants, children, and young adults by pediatricians. Pediatrics. 2003;111:902–7.
Skalet AH, Gombos DS, Gallie BL, Kim JW, Shields CL, Marr BP, et al. Screening children at risk for retinoblastoma: consensus report from the American Association of Ophthalmic Oncologists and Pathologists. Ophthalmology. 2018;125:453–8.
Antoneli CBG, Steinhorst F, de Cássia Braga Ribeiro K, Novaes PER, Chojniak MM, Arias V, et al. Extraocular retinoblastoma: a 13‐year experience. Cancer. 2003;98:1292–8.
Leander C, Fu LC, Pena A, Howard SC, Rodriguez‐Galindo C, Wilimas JA, et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49:817–9.
Antoneli CBG, Steinhorst F, Ribeiro KDCB, Chojniak MM, Novaes PER, Arias V, et al. The pediatrician’s ability to recognize the presenting signs and symptoms of retinoblastoma. Rev da Assocçao Médica Brasileira. 2004;50:400–2.
Nathan PC, Ness KK, Mahoney MC, Li Z, Hudson MM, Ford JS, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;153:442–51.
Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–90.
Yan AP, Chen Y, Henderson TO, Oeffinger KC, Hudson MM, Gibson TM, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: a childhood cancer survivor study. J Clin Oncol. 2020;38:1711–22.
Smith R, Cokkinides V, von Eschenbach A, Levin B, Cohen C, Runowicz C. American Cancer Society Guidelines for the Early Detection of Cancer. CA Cancer J Clin. 2002;52:8–22.
Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. https://doi.org/10.17226/11468.
American College of Surgeons. Important information regarding CoC survivorship care plan standard. https://www.facs.org/quality-programs/cancer/news/survivorship. 2021.
Halton J, Walker E, Greenberg M, Greenberg C. Physician workforce in pediatric oncology: A pediatric oncology group of Ontario (POGO) exercise in establishing the appropriate physician ratio. Pediatr Blood Cancer. 2007;48:626–626.
Halton JM, Hand J, Byron P, Strother D, Blanchette V, C17 Council of Canadian Pediatric Hematology Oncology, T. D. et al. Establishing physician to patient ratios and predicting workforce needs for Canadian pediatric hematology‐oncology programs. Pediatr Blood Cancer. 2013;60:564–9.
Centre for Surveillance and Applied Research, Public Health Agency of Canada. Cancer in Young People in Canada Data Tool. 2020 Edition. Public Health Infobase. Ottawa (ON): Public Health Agency of Canada, 2020.
Dixon SB, Bjornard KL, Alberts NM, Armstrong GT, Brinkman TM, Chemaitilly W, et al. Factors influencing risk‐based care of the childhood cancer survivor in the 21st century. CA Cancer J Clin. 2018;68:133–52.
Kratz CP, Achatz MI, Brugieres L, Frebourg T, Garber JE, Greer M-LC, et al. Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res. 2017;23:e38–e45.
Ballinger ML, Best A, Mai PL, Khincha PP, Loud JT, Peters JA, et al. Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: a meta-analysis. JAMA Oncol. 2017;3:1634–9.
Chompret AS, Abel A, Stoppa-Lyonnet D, Brugières L, Pagès S, Feunteun J, et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet. 2001;38:43.
Brodeur GM, Nichols KE, Plon SE, Schiffman JD, Malkin D. Pediatric cancer predisposition and surveillance: an overview, and a tribute to Alfred G. Knudson Jr. Clin Cancer Res. 2017;23:e1–e5.
Samuel N, Villani A, Fernandez CV, Malkin D. Management of familial cancer: sequencing, surveillance and society. Nat Rev Clin Oncol. 2014;11:723–31.
Green DM. The evolution of treatment for Wilms tumor. J Pediatr Surg. 2013;48:14–9.
Barr RD. “Delays” in diagnosis: a misleading concept, yet providing opportunities for advancing clinical care. J Pediatr Hematol Oncol. 2014;36:169–72.
Worden JW, Weisman AD. Psychosocial components of lagtime in cancer diagnosis. J Psychosom Res. 1975;19:69–79.
National Patient Safety Agency. Delayed diagnosis of cancer: Thematic review. London: National Reporting and Learning Service, 2010.
Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087.
Brasme J-F, Morfouace M, Grill J, Martinot A, Amalberti R, Bons-Letouzey C, et al. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol. 2012;13:e445–e459.
Dang‐Tan T, Franco EL. Diagnosis delays in childhood cancer: a review. Cancer. 2007;110:703–13.
Dobson CM, Russell AJ, Rubin GP. Patient delay in cancer diagnosis: what do we really mean and can we be more specific? BMC Health Serv Res. 2014;14:1–6.
Purkayastha D, O’Hara C, Moran, T. Routes to diagnosis: investigating the different pathways for cancer referrals in England for teenagers and young adults. London: National Cancer Intelligence Network; 2013.
Armstrong L. It’s not about the bike: My journey back to life. Penguin; 2001.
Diorio C, Lam CG, Ladas EJ, Njuguna F, Afungchwi GM, Taromina K, et al. Global use of traditional and complementary medicine in childhood cancer: a systematic review. J Glob Oncol. 2017;3:791–800.
Fern LA, Birch R, Whelan J, Cooke M, Sutton S, Neal RD, et al. Why can’t we improve the timeliness of cancer diagnosis in children, teenagers, and young adults? BMJ. 2013;347:f6493.
Walker DA. Helping GPs to diagnose children’s cancer. Br J Gen Pract. 2021;71:151–2.
Leal‐Leal CA, Dilliz‐Nava H, Flores‐Rojo M, Robles‐Castro J. First contact physicians and retinoblastoma in Mexico. Pediatr Blood Cancer. 2011;57:1109–12.
Yousef YA, AlNawaiseh T, AlJabari R, Muhsen S, Al-Nawaiseh I. Retinoblastoma awareness among first contact physicians in Jordan. Ophthalmic Genet. 2019;40:191–5.
Piramal Foundation. A cancer screening program for rural Telangana. http://www.piramalswasthya.org/wp-content/uploads/2019/03/Cancer-Mobile-Unit-Launch_Swasthya.pdf. 2021.
Nair MK, Varghese C, Mathew B, Sankaranarayanan R. Prevention and early detection of oral, breast and cervical cancers: a practical approach in Indian context. J Indian Med Assoc. 1993;91:94–6.
Noronha V, Tsomo U, Jamshed A, Hai M, Wattegama S, Baral R, et al. A fresh look at oncology facts on south central Asia and SAARC countries. South Asian J Cancer. 2012;1:1.
Wright JG, Menaker RJ. Waiting for children’s surgery in Canada: the Canadian Paediatric Surgical Wait Times project. CMAJ. 2011;183:E559–E564.
Improving outcomes in children and young people with cancer. London: NICE: 2005. https://www.nice.org.uk/guidance/qs55.
Barraclough KNew. NICE guidance on referral for cancer. BMJ. 2015;351:h3640.
Neal R, Din N, Hamilton W, Ukoumunne O, Carter B, Stapley S, et al. Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer. 2014;110:584–92.
Shanmugavadivel D, Liu J-F, Murphy L, Wilne S, Walker D. Accelerating diagnosis for childhood brain tumours: an analysis of the HeadSmart UK population data. Arch Dis Child. 2020;105:355–62.
Abdelkhalek E, Sherief L, Kamal N, Soliman R. Factors associated with delayed cancer diagnosis in egyptian children. Clin Med Insights Pediatr. 2014;8: S16413.
Berhane A, Hailu T, Mulugeta A. Determinants of delayed diagnosis among pediatric cancer patients from Ayder Comprehensive Specialized Hospital, Mekelle, Northern Ethiopia. BMC Pediatr. 2019;19:1–8.
Venkatasai JP, Srinivasamaharaj S, Sneha LM, Scott JX, Baby AK, Rajan M. Pediatric hematological malignancy: identification of issues involved in the road to diagnosis. South Asian J Cancer. 2017;6:028–30.
Verma N, Bhattacharya S. Time to diagnosis and treatment of childhood cancer. Indian J Pediatr. 2020;87:641–3.
Handayani K, Sitaresmi M, Supriyadi E, Widjajanto P, Susilawati D, Njuguna F, et al. Delays in diagnosis and treatment of childhood cancer in Indonesia. Pediatr Blood Cancer. 2016;63:2189–96.
Buckle GC, Collins JP, Sumba PO, Nakalema B, Omenah D, Stiffler K, et al. Factors influencing time to diagnosis and initiation of treatment of endemic Burkitt Lymphoma among children in Uganda and western Kenya: a cross-sectional survey. Infect Agents Cancer. 2013;8:1–16.
Njuguna F, Martijn H, Langat S, Musimbi J, Muliro H, Skiles J, et al. Factors influencing time to diagnosis and treatment among pediatric oncology patients in Kenya. Pediatr Hematol Oncol. 2016;33:186–99.
Chukwu B, Ezenwosu O, Ikefuna A, Emodi I. Diagnostic delay in pediatric cancer in Enugu, Nigeria: a prospective study. Pediatr Hematol Oncol. 2015;32:164–71.
Stefan DC, Siemonsma F. Delay and causes of delay in the diagnosis of childhood cancer in Africa. Pediatr Blood Cancer. 2011;56:80–5.
Fabian ID, Abdallah E, Abdullahi SU, Abdulqader RA, Boubacar SA, Ademola-Popoola DS, et al. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020;6:685–95.
Kaliki S, Ji X, Zou Y, Rashid R, Sultana S, Taju Sherief S, et al. Lag time between onset of first symptom and treatment of retinoblastoma: an international collaborative study of 692 patients from 10 countries. Cancers. 2021;13:1956.
Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Opthalmol. 2009;93:1129–31.
Rodriguez-Galindo C, Friedrich P, Morrissey L, Frazier L. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25:3–15.
Lam CG, Howard SC, Bouffet E, Pritchard-Jones K. Science and health for all children with cancer. Science. 2019;363:1182–6.
Acknowledgements
We are grateful to Dr. Ligia Fu (Hospital Escuela, Tegucigalpa, Honduras), Dr. Carlos Rodriguez-Galindo (St. Jude Children’s Research Hospital, Memphis TN, United States), Dr. Kathy Pritchard-Jones (International Society of Paediatric Oncology, London, United Kingdom), Kathy Brodeur-Robb and Dr. Leah Young (C17 Children’s Cancer and Blood Disorders, Canada) and Dr. Lorna Fern (University College London, United Kingdom) for valuable suggestions and advice.
Funding
CM received an MSc. stipend from the Division of Cancer Epidemiology, McGill University. The other authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualisation, writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to an error in the legend of figure 1
Rights and permissions
About this article
Cite this article
Mullen, C.J.R., Barr, R.D. & Franco, E.L. Timeliness of diagnosis and treatment: the challenge of childhood cancers. Br J Cancer 125, 1612–1620 (2021). https://doi.org/10.1038/s41416-021-01533-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01533-4