Abstract
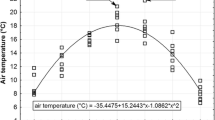
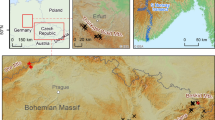
Leaves of European larch, silver birch, and bilberry were sampled 5–7 times per growing season in 2010–2019 in a locality near the city of Litvínov in the Krušné Hory Mts. (Ore Mts.) near the Czech/German border. The locality is characterised by a large amount of plant-available Mn because of acidic soils in the study area. All three investigated plants at the studied site acquired manganese concentrations close to the definition of hyperaccumulation (ca. 10,000 mg kg−1). This paper presents the most detailed collection of plant material for the characterisation of seasonal dynamics of Mn concentrations in the foliage of the three studied plants under field conditions and compares this information with that in published studies. Time (day in the year or day in the growing season) and cumulative precipitation anomalies were major and minor variables, respectively, explaining Mn dynamics in leaves, while temperature and insolation anomalies were not significant. The three investigated species showed plant-specific Mn acquisition rates in the growing season and specific effects of precipitation. Seasonal dynamics must be considered if plant leaves are used for environmental monitoring.





Similar content being viewed by others
Availability of data and material
The datasets generated and/or analysed during the current study are available in Supplementary information or from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Albornoz, F. E., et al. (2021). Revisiting mycorrhizal dogmas: Are mycorrhizas really functioning as they are widely believed to do? Applied Soil Ecology, 3(1), 73–82.
Alriksson, A., & Eriksson, H. M. (2001). Distribution of Cd, Cu, Pb and Zn in soil and vegetation compartments in stands of five boreal tree species in N.E, Sweden. Water, Air, and Soil Pollution, 1, 461–475.
Baker, A. J. M., Reeves, R. D., & Hajar, A. S. M. (1994). Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & Presl (Brassicaceae). New Phytologist, 127, 61–68. https://doi.org/10.1111/j.1469-8137.1994.tb04259.x
Baker, A. J. M., & Brooks, R. R. (1989). Terrestrial higher plants which hyperaccumulate metallic elements – A review of their distribution, ecology and phytochemistry. Biorecovery, 1, 81–126.
Bergmann, W. (1988). Ernährungsstörungen bei Kulturpflanzen. (Entstehung, visuelle und analytische Diagnose). Second ed., Jena: VEB Gustav Fischer Verlag.
Berner, E., Berner, R., & Moulton, K. (2003). Plants and mineral weathering: Present and past. Treatise on Geochemistry, 5, 169–188.
Białońska, D., Zobel, A., Kuraś, M., Tykarska, T., & Sawicka-Kapusta, K. (2007). Phenolic compounds and cell structure in bilberry leaves affected by emissions from a Zn–Pb smelter. Water, Air, and Soil Pollution, 181, 123–133.
Bormann, B. T., Wang, D., Snyder, M. C., Bormann, F. H., Benoit, G., & April, R. (1998). Rapid, plant-induced weathering in an aggrading experimental ecosystem. Biogeochemistry, 43, 129–155.
Boyd, R. S. (2004). Ecology of metal hyperaccumulation. New Phytologist, 162, 563–567.
Brekken, A., & Steinnes, E. (2004). Seasonal concentrations of cadmium and zinc in native pasture plants: Consequences for grazing animals. Science of the Total Environment, 326, 181–195.
Březinová, T., & Vymazal, J. (2015). Evaluation of heavy metals seasonal accumulation in Phalaris arundinacea in constructed treatment wetland. Ecological Engineering, 79, 94–99.
Creighton, L. G., & Spiers, J. M. (1992). Inheritance of tolerance to mineral element-induced chlorosis in rabbiteye blueberry. International Journal of Horticultural Science, 27, 148–151.
Drever, J. I. (1994). The effect of land plants on weathering rates of silicate minerals. Geochimica Et Cosmochimica Acta, 58, 2325–2332.
Ducic, T., & Polle, A. (2005). Transport and detoxification of manganese and copper in plants. Brazilian Journal of Plant Physiology, 17, 103–112.
EC-UN/ECE, Stefan. K., Fürst, A., Hacker, R., Bartels, U. (1995). Forest foliar condition in Europe - Results of large-scale foliar chemistry surveys. EC, UN/ECE.
Fageria, N. K., Stone, L. F. (2008). Micronutrient deficiency problems in South America. In: Alloway, B. J. (ed.), Micronutrient deficiencies in global crop production. Springer, Dordrecht.
Fernando, D. R., et al. (2013). Microbeam methodologies as powerful tools in manganese hyperaccumulation research: Present status and future directions. Frontiers of Plant Science, 4.
Fernando, D. R., & Lynch, J. P. (2015). Manganese phytotoxicity: New light on an old problem. Annals of Botany, 116, 313–319.
Fiala, P., Reininger, D., Samek, T., Němec, P., & Sušil, A. (2013). A survey of nutrition forest in the Czech Republic, 1996–2011. Brno: Central Institute for Supervising and Testing in Agriculture.
Forest management plan. (2010). Forest management plan: Litvínov city forests 2010–2019. Text part. Ekoles–Projket s.r.o., Jablonec nad Nisou.
Foy, C. D., Fleming, A. L., & Armlger, W. H. (1969). Differential tolerance of cotton varieties to excess manganese. Agronomy Journal, 61, 690–694.
Goldschmidt, V. M. (1937). The principles of distribution of chemical elements in minerals and rocks. Journal of the Chemical Society, 655–673.
Goss, M. J., & Carvalho, M. J. (1992). Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant and Soil, 139, 91–98.
Gransee, A., & Führs, H. (2013). Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant and Soil, 368, 5–21.
Heal, K. V. (2000). Manganese and land-use in upland catchments in Scotland. Science of the Total Environment, 265, 169–179.
Heenan, D., & Campbell, L. (1981). Influence of potassium and manganese on growth and uptake of magnesium by soybeans (Glycine max (L.) Merr. Cv. Bragg). Plant and Soil, 61, 447–456.
Heinrichs, H., & Mayer, R. (1980). The role of forest vegetation in the biogeochemical cycle of heavy metals. Journal of Environmental Quality, 9, 111–118.
Herndon, E., Yarger, B., Frederick, H., & Singer, D. (2019). Iron and manganese biochemistry in forested coal mine spoil. Soil Systems, 3, 13. https://doi.org/10.3390/soilsystems3010013
Hrdlička P., Kula E. (1998). Element content in leaves of birch (Betula verrucosa Ehrh.) in an air polluted area. Trees, 13 (2), 68–73.
Hrdlička, P., & Kula, E. (2004). Changes in the chemical content of birch (Betula pendula Roth) leaves in the air polluted Krušné hory mountains. Trees, 18, 237–244.
Hrdlička, P., & Kula, E. (2010). Changes in element content of birch leaves (Betula pendula Roth) in polluted air. Polish J. of Environ. Stud., 20(3), 661–667.
Joslin, J. D., Kelly, J. M., & Van Mlegroet, H. (1992). Soil chemistry and nutrition of North American spruce-fir stands: Evidence for recent change. Journal of Environmental Quality, 21, 1250.
Juice, S. M., Fahey, T. J., Siccama, T. G., Driscoll, C. T., Denny, E. G., & Eagar, C. (2006). Response of sugar maple to calcium addition to Northern Hardwood Forest. Ecology, 87, 1267–1280.
Kabata-Pendias, A. (2011). Trace elements in soil and plants (Fourth ed.). Boca Raton: CRC Press 520 pp.
Kandziora-Ciupa, M., Ciepał, R., Nadgórska-Socha, A., et al. (2013). A comparative study of heavy metal accumulation and antioxidant responses in Vaccinium myrtillus L. leaves in polluted and non-polluted areas. Environmental Science and Pollution Research, 20, 4920–4932.
Kandziora-Ciupa, M., Nadgórska-Socha, A., Barczyk, G., et al. (2017). Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology, 26, 966–980.
Kim, H. T., & Kim, J. G. (2018). Seasonal variations of metal (Cd, Pb, Mn, Cu, Zn,) accumulation in a voluntary species, Salix subfragilis, in unpolluted wetlands. Science of the Total Environment, 610–611, 1210–1221. https://doi.org/10.1016/j.scitotenv.2017.08.137
Kitao, M., Lei, T. L., & Koike, T. (1997). Effects of manganese toxicity on photosynthesis of white birch (Betula platyphylla var. japonica) seedlings. Physiologia Plantarum, 101, 249–256.
Kogelmann, W. J., & Sharpe, W. E. (2006). Soil acidity and manganese in declining and non-declining sugar maple stands in Pennsylvania. Journal of Environmental Quality, 35, 433–441.
Kochenderfer, J. N., Helvey, J. D. (1989). Hydrologic impacts of mechanized site preparation in the central Appalachians. In: Kochendetfer, J. N., Crews, J. T., Smith, H. C. Effects of fertilization on the growth and development of a Japanese larch plantation in West Virginia: The 7th central hardwood forest conference: 1995 March 5–8; Carbondale, IL. Res. Pap. NE- 700. U. S. Department of Agriculture, Forest Service.
Kochian, L. V., Hoekenga, O. A., & Pineros, M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminium tolerance and phosphorus efficiency. Annual Review of Plant Biology, 55, 459–493.
Kozanecka, T., Chojnicki, J., & Kwasowski, W. (2002). Content of heavy metals in plant from pollution-free regions. Polish Journal of Environmental Studies, 11(4), 395-400.
Kula, E., Hrdlička, P., Hedbávný, J., & Švec, P. (2012). Various content of manganese in selected forest tree species and plants in the undergrowth. Beskydy, 5(1), 19–26.
Kula, E., Martinek, P., Chromcová, L., & Hedbávný, J. (2014). Development of gypsy moth (Lymantria dispar L.) affected by manganese in food. Environmental Science and Pollution Research, 21, 11987–11997.
Kula, E., Wildová, E., Hrdlička, P. (2018). Accumulation and dynamics of manganese content in bilberry (Vaccinium myrtillus L.). Environmental Monitoring and Assessment, 190–224. https://doi.org/10.1007/s10661-018-6604-8
Laboratory-Morava. (2010). Standardized internal laboratory methods for chemical analyses soil and plant (p. 30). Studénka: Laboratory Morava, Inc., accredited laboratory.
Lambers, H., et al. (2015). Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends in Plant Science, 20, 83–90.
Lambers, H., Oliveira, R. S. (2019). Plant physiological ecology, 3rd ed. Springer, Cham. https://doi.org/10.1007/97830-302-9639-1
Lambers, H., Wright, I. J., Guilherme Pereira, C., et al. (2021). Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant and Soil, 461, 43–62. https://doi.org/10.1007/s11104-020-04690-2
Landre, A. L., & Watmough, S. A. (2010). Metal pools, fluxes, and budgets in an acidified forested catchment on the precambrian shield, Central Ontario, Canada. Water, Air, and Soil Pollution, 209, 209–228.
Lavres Junior, J., Reis, A. R., Rossi, M. L., Cabral, C. P., Nogueira, N. L., & Malavolta, E. (2010). Changes in the ultrastructure of soybean cultivars in response to manganese supply in solution culture. Science in Agriculture, 67, 287–294.
Lei, Y., Korpelainen, H., & Li, C. (2007). Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere, 65, 686–694.
Lu, X. K., Mo, J. M., Gilliam, F. S., Zhou, G. Y., & Fang, Y. T. (2010). Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Global Change Biology, 16(10), 2688–2700.
Marschner, H. (1995). Mineral nutrition of higher plants (2nd ed.). Academic Press.
Martinek, P., Kula, E., & Hedbavný, J. (2017). Reaction of leaf weevil Phyllobius arborator (Coleoptera, Curculionidae) to manganese content in diet Environmental. Environmental Entomology, 46(1), 131–136.
Martinek, P., Kula, E., & Hedbavný, J. (2018). Reaction of Melolontha hippocastani adults to high manganese content in food. Ecotoxicology and Environmental Safety, 148, 37–43.
Martínek, P., Hedvábný, J., Kudláček, T., Šťasta, M., & Kula, E. (2020). Adverse responses on Cabera pusaria caterpillars to high dietary manganese concentration. Entomol. Exp. Et Appl., 168, 635–643.
McMeans, B. C., McCann, K. S., Humphries, M., Rooney, N., & Fisk, A. T. (2015). Food web structure in temporally-forced ecosystems. Trends in Ecology & Evolution, 30(October), 662–672.
Mengel, K., & Kirkby, E. A. (2001). Principles of plant nutrition (5th ed.). Kluwer Academic Publishers.
Migeon, A., Richaud, P., Guinet, F., Chalot, M., & Blaudez, D. (2009). Metal accumulation by woody species on contaminated sites in the north of France. Water, Air, and Soil Pollution, 204, 89–101.
Mróz, L., Demczuk, M. (2010). Contents of phenolics and chemical elements in bilberry (Vaccinium myrtillus L.) leaves from copper smelter area. Polish Journal of Ecology, 58(3), 475–486.
Navrátil, T., Shanley, J. B., Skřivan, P., Krám, P., Mihaljevič, M., & Drahota, P. (2007). Manganese biogeochemistry in a central Czech Republic catchment. Water, Air, and Soil Pollution, 186, 149–165.
Ohki, K. (1984). Manganese deficiency and toxicity effects on growth, development, and nutrient composition in wheat. Agronomy Journal, 76, 213–218.
Oliva, M., José Vicente, J., & Gravato, C., Guilhermino, L., Dolores Galindo- Riaño, M. (2012). Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): Seasonal and spatial variation. Ecotoxicology and Environmental Safety, 75(1), 151–162.
Pickens, C. J., Sharpe, W. E., & Edwards, P. J. (1995). The effects of doubling annual N and S deposition on foliage and soil chemistry and growth of Japanese larch (Larix leptolepis Sieb. and Zucc.) in north central West Virginia. In: Gottschalk, Kurt W.; Fosbroke, Sandra LC, ed. Proceedings, 10th Central Hardwood Forest Conference; 1995 March 5-8; Morgantown, WV.: Gen. Tech. Rep. NE-197. Radnor, PA: US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station. 131-140(Vol. 197).
Power, M. E., Parker, M. S., & Dietrich, W. E. (2008). Seasonal reassembly of a river food web: Floods, droughts, and impacts of fish. Ecological Monographs, 78(2), 263–282.
Reeves, R. D. (2006). Hyperaccumulation of trace elements by plants. In J.-L. Morel, G. Echevarria, & N. Goncharova (Eds.), Phytoremediation of metal-contaminated soils. NATO Science, 68, 25–52.
Reeves, R. D., & Baker, A. J. M. (2000). Metal – Accumulating plants. In I. Raskin & B. D. Ensley (Eds.), Phytoremediation of toxic metals: Using plants to clean-up the environment (pp. 193–230). John Wiley and Sons.
Reimann, C., Fabian, K., Flem, B., Andersson, M., Filzmoser, P., & Englmaier, P. (2018). Geosphere-biosphere circulation of chemical elements in soil and plant systems from a 100 km transect from southern central Norway. Science of the Total Environment, 639, 129–145.
Reimann, C., Koller, F., Frengstad, B., Kashulina, G., Niskavaara, H., & Englmaier, P. (2001). Comparison of the element composition in several plant species and their substrate from a 1500000-km2 area in Northern Europe. Science of the Total Environment, 278, 87–112.
Reimann, C., Arnoldussen, A., Finne, T., Koller, R., & Englmaier, P. (2007a). Element contents in leaves of four plant species (birch, mountain ash, fern and spruce) along anthropogenic and geogenic concentration gradients. Science of the Total Environment, 377, 416–433.
Reimann, C., Arnoldussen, A., Englmaier, P., Filzmoser, P., Finne, T. E., Garret, R. G., et al. (2007). Element concentrations and variations along 120-km transect in southern Norway – Anthropogenic vs. geogenic vs. biogenic element sources and cycles. Applied Geochemistry, 22, 851–871.
Salemaa, M., Derome, J., Helmisaari, H. S., Nieminen, T., & Vanha-Majamaa, I. (2004). Element accumulation in boreal bryophytes, lichens and vascular plants exposed to heavy metal and sulphur deposition in Finland. Science of the Total Environment, 324, 141–160.
Sanderson, K., Jordan, C., & Fillmore, S. (2008). Leaf nutrient ranges for wild blueberries in Prince Edward Island. International Journal of Fruit Science, 8, 63–68.
Sembratowicz, I., Rusinek, E., Ognik, K., Truchlinsky, J. (2009). Concentrations of trace elements and heavy metals at selected medicinal plants harvested in two vegetation periods. Department of Biochemistry and Toxicology. University of Life Sciences, 55 (1), 22–28.
Schlegel, H., Amundson, R. G., & Huttermann, A. (1992). Element distribution in red spruce (Pica rubens) fine roots; evidence for aluminium toxicity at Whiteface Mountain. Canadian Journal of Forest Research, 22, 1132–1138.
Schweitzer, C. J., Sharpe, W. E., Edwards, P. J. (1999). The effect of soil manganese on Japanese Larch (Larix leptolepis Sieb. And Zucc.) Seedlings in the greenhouse. In: Stringer, Jeffrey W.; Loftis, David L., eds. 1999. Proceedings, 12th central hardwood forest conference; 1999 February 28-March 1–2; Lexington, KY. Gen. Tech. Rep. SRS-24. Asheville, NC: U. Department of Agriculture, Forest Service, Southern Research Station.
Skřivan, P., Navrátil, T., Vach, M., Sequens, J., Kurian, M., & Kvidova, O. (2002). Biochemical cycles of metals in the environment: Factors controlling their content in the tissues of selected forest tree species. Scientia Agriculturae Bohemica, 2, 71–78.
Sobíšek, B., Munzar, J., Krška, K., et al. (1993). Meteorological Dictionary Interpretation & Terminology. Academia.
Taylor, L. L., Leake, J. R., Quirk, J., Hardy, K., Banwart, S. A., & Beerling, D. J. (2009). Biological weathering and the long-term carbon cycle: Integrating mycorrhizal evolution and function into the current paradigm. Geobiology, 7, 171–191.
Terry, N., Evans, P. S., & Thomas, D. E. (1975). Manganese toxic effects on leaf cell multiplication and expansion and on dry matter yield of sugar beets. Crop Science, 15, 205–208.
Thornton, F. C., Schaedle, M., & Raynal, D. J. (1989). Tolerance of red oak and American and European beech seedlings to aluminium. Journal of Environmental Quality, 18, 541–545.
Verbruggen, N., & Herma ns, C., Schat, H. . (2009). Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist, 181, 759–776.
Viers, J., Prokushkin, A. S., Pokrovsky, O. S., et al. (2013). Seasonal and spatial variability of elemental concentrations in boreal forest larch foliage of Central Siberia on continuous permafrost. Biogeochemistry, 113, 435–449. https://doi.org/10.1007/s10533-012-9770-8
Vitória, A. P., da Silva Santos, J. L., Barros Salomao, M. S. M., de Oliveira Vieira, T., Da Cunha, M., Pireda, S. F., & Rabelo, G. R. (2015). Influence of ecologic type, seasonality, and origin of macrophyte in metal accumulation, anatomy and ecophysiology of Eichhornia crassipes and Eichhornia azurea. Aquatic Botany, 125, 9–16.
White, E. R. (2020). Seasonality in ecology: Progress and prospects in theory. Ecological Complexity, 44, 100867.
White, E. R. (2019). Minimum time required to detect population trends: The need for long-term monitoring programs. BioScience, 69(1), 40–46.
Wislocka, M., Krawczyk, J., Klink, A., & Morrison, L. (2006). Bioaccumulation of heavy metals by selected plants species from uranium mining dumps in the society MTS Poland. Polish Journal of Environmental Studies, 15(5), 811–818.
Wissemeier, A. H., & Horst, W. J. (1992). Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata L. Walp.). Plant and Soil, 143, 299–309.
Wu, S. (1994). Effect of manganese excess on the soybean plant cultivated under various growth conditions. Journal of Plant Nutrition, 17, 993–1003.
Acknowledgements
The authors thank T. Matys Grygar (Faculty of Environment, J. E. Purkyně University in Ústí nad Labem, and Institute of Inorganic Chemistry CAS, Řež) for discussions and help with manuscript preparation. The authors also thank a student Gabriela Bilkova for her help within filedworks and samples preparation.
Funding
The presented paper was supported by grant UJEP-SGS-2020–44-005–3 provided as part of a student grant competition at Jan Evangelista Purkyně University in Ústí nad Labem (Czech Republic) and by grant UJEP-IGA-TC-2019–44-02–2 provided by the Internal Grant Agency of the same institution. The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz (project No. LM2015073) and by project INVUST (CZ.02.1.01/0.0/0.0/16_017/0002678), supported by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the conception and design of the manuscript, acquisition of data, analysis and interpretation of the data, revisions, and final approval of the version to be submitted for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Bilberry, larch, and birch are Mn-accumulating species.
• Seasonality is essential for determining element intake in plants.
• Environmental monitoring should include several samplings per season.
• Leaves on Mn-tolerant species accumulate more Mn under acidic soil conditions.
• Precipitation affects manganese uptake by plants.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wildová, E., Elznicová, J. & Kula, E. Seasonal dynamics of manganese accumulation in European larch (Larix decidua Mill.), silver birch (Betula pendula Roth), and bilberry (Vaccinium myrtillus L.) over 10 years of monitoring. Environ Monit Assess 193, 612 (2021). https://doi.org/10.1007/s10661-021-09415-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09415-1