Abstract
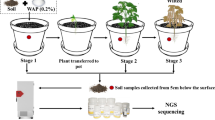
Plants release various metabolites from roots and root exudates contribute to differences in stress tolerance among plant species. Plant and soil microbes have complex interactions that are affected by biotic and abiotic factors. The purpose of this study was to examine the differences in metabolites in root exudates of rice (Oryza sativa) cultivars and their correlation with bacterial populations in the rhizosphere. Two rice cultivars (O. sativa cv. Akamai and O. sativa cv. Koshihikari) were grown in soils fertilized with 0 g P kg−1 (− P) or 4.8 g P kg−1 (+ P). Root exudates and root-attached soil were collected at 13 and 20 days after transplanting (DAT) and their metabolites and bacterial community structure were determined. The exudation of proline, serine, threonine, valine and 4-coumarate were increased under low P conditions in both cultivars. There was a positive correlation between the concentration of pantothenate in root exudates and the representation of members of the genera Clostridium and Sporosarcina, which were negatively correlated with root dry weight. Gracilibacter, Opitutus, Pelotomaculum, Phenylobacterium and Oxobacter were positively correlated with root dry weight and presence of allantoin, 2-aminobtyrate and GlcNac. This study provides new information about the response of plants and rhizosphere soil bacteria to low P conditions.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Ankati S, Podile AR (2019) Metabolites in the root exudates of groundnut change during interaction with plant growth promoting rhizobacteria in a strain-specific manner. J Plant Physiol. https://doi.org/10.1016/j.jplph.2019.153057
Annan FJ, Al-Sinawi B, Humphreys CM, Norman R, Winzer K, Kopke M, Simpson SD, Minton NP, Henstra AM (2019) Engineering of vitamin prototrophy in Clostridium ljungdahlii and Clostridium autoethanogenum. Appl Microbiol Biotechnol 103:4633–4648
Badri DV, Chaparro JM, Zhang RF, Shen QR, Vivanco JM (2013) Application of natural blends of phytochemicals derived from the root exudates of arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem 288:4502–4512
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H, Huerta-Cepas J, Medema MH, Maltz MR, Mundra S, Olsson PA, Pent M, Polme S, Sunagawa S, Ryberg M, Tedersoo L, Bork P (2018) Structure and function of the global topsoil microbiome. Nature 560(7717):233–237
Bargaz A, Lyamlouli K, Chtouki M, Zeroual Y, Dhiba D (2018) Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front Microbiol 9:1606
Barthelmebs L, Divies C, Cavin JF (2000) Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl Environ Microbiol 66:3368–3375
Bisanz JE (2018) qiime2R: importing QIIME2 artifacts and associated data into R sessions. https://github.com/jbisanz/qiime2R.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wiren N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, P, potassium, and iron deficiency. J Plant Nutr Soil Sci 174:3–11
Cordell D, Drangert JO, White S (2009) The story of P: global food security and food for thought. Global Environ Change 19:292–305
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
Devendran S, Shrestha R, Alves JMP, Wolf PG, Ly L, Hernandez AG, Mendez-Garcia C, Inboden A, Wiley J, Paul O, Allen A, Springer E, Wright CL, Fields CJ, Daniel SL, Ridlon JM (2019) Clostridium scindens ATCC 35704: integration of nutritional requirements, the complete genome sequence, and global transcriptional responses to bile acids. Appl Environ Microbiol. https://doi.org/10.1128/AEM.00052-19
Ding LJ, Su JQ, Xu HJ, Jia ZJ, Zhu YG (2015) Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-C-13-acetate probing coupled with pyrosequencing. Isme J 9:721–734
Dinkelaker B, Romheld V, Marschner H (1989) Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ 12:285–292
Dissanayaka D, Maruyama H, Nishida S, Tawaraya K, Wasaki J (2017) Landrace of japonica rice, Akamai exhibits enhanced root growth and efficient leaf P remobilization in response to limited P availability. Plant Soil 414:327–338
Feigel BJ, Knackmuss HJ (1988) Bacterial catabolism of sulfanilic acid via catechol-4-sulfonic acid. Fems Microbiol Lett 55:113–117
Fuchs G (2008) Anaerobic metabolism of aromatic compounds incredible anaerobes. Ann NY Acad Sci 1125:82–99
Gilbert N (2009) Environment: the disappearing nutrient. Nature 461:716–718
Griffiths BS, Ritz K, Ebblewhite N, Dobson G (1999) Soil microbial community structure: effects of substrate loading rates. Soil Biol Biochem 31:145–153
Hocking PJ (2001) Organic acid exuded from roots in phosphorus uptake and aluminium tolerance of plants in acid soils. Adv Agron 74(74):63–97
Hoffland E, Gunter R, Findenegg R, Nelemans JA (1989) Solubilization of rock phosphate by rape II. Local root exudation of organic acids as a response to P-starvation. Plant Soil 113:161–165
Hoffland E, Wei CZ, Wissuwa M (2006) Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283(1–2):155–162
Johnson JF, Vance CP, Allan DL (1996) P deficiency in Lupinus albus—altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase. Plant Physiol 112:31–41
Kirk GJD, Santos EE, Findenegg GR (1999) Phosphate solubilization by organic anion excretion from rice (Oryza sativa L.) growing in aerobic soil. Plant Soil 211(1):11–18
Lahti L, Shetty S (2019) microbiome R package. http://microbiome.github.io
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy-metabolism—organic-carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49(49):219–286
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5(2):169–172
Marschner H, Dell B (1994) Nutrient-uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
McMurdie P, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8(4):e61217
Ohwaki Y, Hirata H (1992) Differences in carboxylic-acid exudation among p-starved leguminous crops in relation to carboxylic-acid contents in plant-tissues and phospholipid level in roots. Soil Sci Plant Nutr 38:235–243
Pennanen T, Caul S, Daniell TJ, Griffiths BS, Ritz K, Wheatley RE (2004) Community-level responses of metabolically-active soil microorganisms to the quantity and quality of substrate inputs. Soil Biol Biochem 36(5):841–848
Peters NK, Frost JW, Long SR (1986) A plant flavone, luteolin, induces expression of rhizobium-meliloti nodulation genes. Science 233:977–980
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J (2010) Strategies for improving P acquisition efficiency of crop plants. Field Crops Res 117:169–176
Reuter DJ, Edwards DG, Wilhelm NS (1997) Temperate and tropical crops. In: Reuter DJ, Robinson JB (eds) Plant analysis: an interpretation manual. CSIRO Publishing, Collingwood, pp 81–284
RStudio Team (2020) RStudio: Integrated development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Schachtman DP, Reid RJ, Ayling SM (1998) P uptake by plants: from soil to cell. Plant Physiol 116:447–453
Segura D, Mahadevan R, Juarez K, Lovley DR (2008) Computational and experimental analysis of redundancy in the central metabolism of Geobacter sulfurreducens. Plos Comput Biol 4:e36
Shen JB, Yuan LX, Zhang JL, Li HG, Bai ZH, Chen XP, Zhang WF, Zhang FS (2011) P dynamics: from soil to plant. Plant Physiol 156:997–1005
Spohn M, Ermak A, Kuzyakov Y (2013) Microbial gross organic P mineralization can be stimulated by root exudates—a P-33 isotopic dilution study. Soil Biol Biochem 65:254–263
Tadano T, Sakai H (1991) Secretion of acid-phosphatase by the roots of several crop species under p-deficient conditions. Soil Sci Plant Nutr 37:129–140
Tawaraya K, Horie R, Saito A, Shinano T, Wagatsuma T, Saito K, Oikawa A (2013) Metabolite profiling of shoot extracts, root extracts, and root exudates of rice plant under p deficiency. J Plant Nutr 36:1138–1159
Tawaraya K, Horie R, Shinano T, Wagatsuma T, Saito K, Oikawa A (2014) Metabolite profiling of soybean root exudates under P deficiency. Soil Sci Plant Nutr 60:679–694
Tawaraya K, Honda S, Cheng W, Chuba M, Okazaki Y, Saito K, Oikawa A, Maruyama H, Wasaki J, Wagatsuma T (2018) Ancient rice cultivar extensively replaces phospholipids with non-P glycolipid under P deficiency. Physiol Plant 163:297–305
Wang P, Kong CH, Hu F, Xu XH (2007) Allantoin involved in species interactions with rice and other organisms in paddy soil. Plant Soil 296:43–51
Watanabe M, Kusano M, Oikawa A, Fukushima A, Noji M, Saito K (2008) Physiological roles of the beta-substituted alanine synthase gene family in arabidopsis. Plant Physiol 146(1):310–320
Yang XF, Li L, Xu Y, Kong CH (2018) Kin recognition in rice (Oryza sativa) lines. New Phytol 220(2):567–578
Yuwono T (2005) Metabolism of betaine as a carbon source by an osmotolerant bacterium isolated from the weed rhizosphere. World J Microbiol Biotechnol 21:69–73
Zhu XF, Wang ZW, Wan JX, Sun Y, Wu YR, Li GX, Shen RF, Zheng SJ (2015) Pectin enhances rice (Oryza sativa) root P remobilization. J Exp Bot 66:1017–1024
Zhu XF, Zhu CQ, Zhao XS, Zheng SJ, Shen RF (2016) Ethylene is involved in root P remobilization in rice (Oryza sativa) by regulating cell-wall pectin and enhancing phosphate translocation to shoots. Ann Bot 118:645–653
Acknowledgements
This study was partly supported by a Grant-in-Aid for Scientific Research (No. 15H04466) from the Japan Society for the Promotion of Science (JSPS), the research project “Development of mitigation and adaptation techniques to global warming in the sectors of agriculture, forestry, and fisheries” funded by the Ministry of Agriculture, Forestry and Fisheries (MAFF), and Japan Advanced Plant Science Network, Japan.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CM and MS contributed equally to this research. Material preparation, data collection, and analysis were performed by CM, MS, and AK. The first draft of the manuscript was written by CM, MS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsushima, C., Shenton, M., Kitahara, A. et al. Multiple analysis of root exudates and microbiome in rice (Oryza sativa) under low P conditions. Arch Microbiol 203, 5599–5611 (2021). https://doi.org/10.1007/s00203-021-02539-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02539-5