Abstract
Acinetobacter baumannii has become a major concern for scientific attention due to extensive antimicrobial resistance. This resistance causes an increase in mortality rate because strains resistant to antimicrobial agents are a major challenge for physicians and healthcare workers regarding the eradication of either hospital or community-based infections. These strains with emerging resistance are a serious issue for patients in the intensive care unit (ICU). Antibiotic resistance has increased because of the acquirement of mobile genetic elements such as transposons, plasmids, and integrons and causes the prevalence of multidrug resistance strains (MDR). In addition, an increase in carbapenem resistance, which is used as last line antibiotic treatment to eliminate infections with multidrug-resistant Gram-negative bacteria, is a major concern. Carbapenems resistant A. baumannii (CR-Ab) is a worldwide problem. Because these strains are often resistant to all other commonly used antibiotics. Therefore, pathogenic multi-drug resistance A. baumannii (MDR-Ab) associated infections become hard to eradicate. Plasmid-mediated resistance causes outbreaks of extensive drug-resistant. A. baumannii (XDR-Ab). In addition, recent outbreaks relating to livestock and community settings illustrate the existence of large MDR-Ab strain reservoirs within and outside hospital settings. The purpose of this review, proper monitoring, prevention, and treatment are required to control (XDR-Ab) infections. Attachment, the formation of biofilms and the secretion of toxins, and low activation of inflammatory responses are mechanisms used by pathogenic A. baumannii strain. This review will discuss some aspects associated with antibiotics resistance in A. baumannii as well as cover briefly phage therapy as an alternative therapeutic treatment.
Similar content being viewed by others
Introduction
Acinetobacter baumannii (A. baumannii) is gram-negative, aerobic coccobacilli, non-motile. A. baumannii belongs to “ESKAPE” six pathogens with multidrug resistance and virulence. This group is a responsible majority of nosocomi infections and can avoid biocidal effect of antimicrobial agents. A. baumannii can be identified by using 16 s ribosomal RNA as well as conserved region of seven housekeeping genes: gltA, gyrB, gdhB, recA, cpn60, gpi, and rpoD by using multilocus sequence typing (MLST) [1,2,3]. Infections of A. baumannii have been considered a major concern because it shows extensive resistance to antibiotics and high molarity death associated with its infections [4,5,6]. Hospitalized and vulnerable patients are at higher risk of A. baumannii infections because it penetrates through skin and airway defects. Furthermore, most infections caused by this bacterium affect patients staying in the intensive care unit (ICU) [7,8,9,10]. A. baumannii causes many infections including skin and soft tissues, wound infections, bacteremia, endocarditis, urinary tract infections (UTIs), meningitis, and pneumonia [8,9,10]. The most common nosocomial infection associated with A. baumannii is pneumonia mainly in patients admitted to ICU and breathing through the ventilator. The mortality rate from A. baumannii caused ventilator-associated pneumonia (VAP) varies from 40 to 70% [11, 12]. Some risk factors related to acute myocardial infarction involve bloodstream infections, immunosuppression, artificial ventilation, preceding antibiotic treatment, and invasive virus colonization [13]. The rate of mortality Infections of the A. baumannii bloodstream range from 28 to 43% [14]. Together with other important agents, A. baumannii interferes with the development of burn infections, where the existence of Multi-Drug Resistance (MDR) strains and low penetration of many antibiotics are the main problems for chemotherapy [15,16,17,18]. The rate of burn infections associated with A. baumannii is about 22% and mainly spread among military personnel, with an MDR rate of about 53% [19]. Additionally, A. baumannii can cause infections related to the central nervous system (CNS) that can be treated by colistin antibiotics [20]. Strains of A. baumannii can survive for long periods in the environment, which assist in the contribution of their dissemination and spread of pathogenic MDR strains. The increase in usages of β-Lactam antibiotics has contributed to the emergence of drug-resistant and rapid development of A. baumannii resistant strains. Infections caused by these resistant strains are treated with carbapenems. However, the emergence and spread of resistant A. baumannii (CR-Ab) to carbapenems has limited the effectiveness of this drug. Furthermore, the emergence of colistin resistant A. baumannii (Col-R-Ab) strains have been recorded and this resistance is occurred due to changes in the structure of the lipo-polysaccharide (LPS). Colistin resistance is showed to occur due to mutations in lpxA/D/C and pmrA/B genes resulting in regulation downwards and modification of lipid A biosynthesis [21, 23]. Moreover, another resistance mechanism for colistin involves plasmid-carryin three genes (mcr-1, mcr-2 and mcr-3) and recent study isolated a novel plasmid carrying mcr-4.3 gene [22, 23]. Since mcr-1 first described from Escherichia coli, many mcr- genes have been identified among Enterobacteriaceae strains include: mcr-1, mcr-2, mcr-3, mcr-4, mcr-5, mcr-6, mcr-7, mcr-8 and mcr-10 but only mcr1, mcr-2, mcr3 and mcr-4 identified in A. baumannii [23]. Infections caused by extensively drug-resistant A. baumannii (XDR-Ab) and plasmid/chromosomal-mediated Col-R-Ab are the main challenge and need a well-planned control program and proper treatment [24]. The outbreaks are critical to the livestock and community that recently have highlighted the existence of large reservoirs of MDR-Ab strains [25, 26].
The antibiotic resistance in A. baumannii
Acinetobacter baumannii strains can develop multiple antibiotic resistance mechanisms, which cause a major health problem in immuno-compromised patients. These strains showed resistance to broad-spectrum β-lactams antibiotics involved carboxy-pencillins, the third generation of cephalosporins, and most recent carbapenems resistance. Furthermore, these strains can produce various aminoglycoside-modifying enzymes and most of these enzymes are related to fluoroquinolones resistance [10, 27]. Moreover, colistin-resistant isolates have been emerged, which restrict the choices of chemotherapy treatment for these isolates. [21, 22]. Pan-β-lactam-resistant baumannii spreads widely and this gives pieces of evidence to the high ability of these bacteria to acquire different mechanisms of resistance as below:
Oxacillinases (class D β-lactamases)
Oxacillinases are specific B-lactamases, which are classified to many groups depending on structural and biochemical properties [7, 28]. The OXA-type group is primarily indicated in Acinetobacter spp. and Pseudomonas aeruginosa. These enzymes can hydrolyze methicillin, amoxicillin, and some antibiotics belong to cephalosporins. Furthermore, the hydrolysis activity of OXA-type group is more effective against oxacillin than benzyl penicillin. Generally, OXA-type enzymes are not considered ESBLs because they cannot hydrolyze the board spectrum cephalosporin, although there are some exceptions, such as OXA-20 in P. aeruginosa and OXA-37 in A. baumannii [29].
Around 350 OXA enzymes (see https://www.lahey.org/studies/webt.asp) have been identified depending on the surface protein recently. The class D of oxacillinases are both chromosomally and plasmid-mediated encoded enzymes [30]. The first discovery of blaOXA-5 was in P. aeruginosa, while blaOXA-23-like blaOXA-51-like, and blaOXA-134 enzymes are isolated from Acinetobacter radioresistance, A. baumannii, and A. lwoffi respectively [5, 31].
bla OXA-23-like oxacillinases
These enzymes were first carbapenem-resistant OXA-type β-lactamases identified among A. baumannii strains, in Scotland and renamed later as blaOXA-23-like enzymes based on their sequence. The First description of the gene encoding these enzymes was in 1985 within a plasmid isolated from A. baumannii strain, which showed MIC level = 16 mg/L for Imipenem [32]. blaOXA-27 enzyme was isolated in Singapore, which shared 99% homology to a blaOXA-23. Moreover, another type of these enzyme was identified in China known as blaOXA-49, which is isolated from carabpenem resistant Acinetobacter spp., [33].
Later, the blaOXA-23 was detected from A. baumannii throughout the world such as Brazil, China, London, and Singapore and these enzymes are encoded by chromosomal genes or located on plasmids [34, 35]. In vivo, blaOXA-23-like enzyme confers the antibiotics resistance mechanism to ticarcelin, meropenem, amoxicillin, and imipenem. In a study, a new strain acquired a plasmid containing the blaOXA-23-like gene showed a MIC ranged from moderate to high levels, which equals to 16 to 32 mg/L against carbapenems and led to overexpression of Ade-ABC efflux pump, which refers to involvement other genetic factors associated related to carbapenam resistance of the blaOXA-23-like gene [36].
Several bla OXA-23-like enzymes were discovered including bla OXA-27, bla OXA-102, bla OXA-103, bla OXA-105 and bla OXA-133, In addition, searching BLAST tool in NCBI web shows several other related enzymes including blaOXA-146 (FJ194494), blaOXA-168 (HM488990), blaOXA-169 (HM488991), blaOXA-170 (HM488992), blaOXA-171 (HM488992) and blaOXA-225 (JN638887) genes. In addition, the blaOXA-33 was identified in A. radioresistance [37].
The blaOXA-134 enzyme has a 63 percent amino acid identity with the blaOXA-23 enzyme and it inherently presents in A. lwoffi. A gene like blaOXA-23 has also been detected with the chromosomal DNA in Proteus mirabilis. The blaOXA-73 was discovered in Klebsiella pneumoniae [38]. Isolation CR-Ab with blaOXA-23-like gene has been found in South American and Southeast Asia [39, 40]. In a study conducted in Columbia, blaOXA-23-like was found in 65/66 isolates of CR-Ab strains and showed the presence of a common clone in two hospitals in the city [39]. In China, it was noticed an increase in carbapenem resistance rate within training hospitals from 5 and 50% in the ICU over a 6-year period. 97.7% of these ratios belonged to CR-Ab contains the blaOXA-23-like. Furthermore, a research in China identified major carriers for spread of the blaOXA-23-like among A. baumanniii include; Tn2008 transposon, which blaOXA-23-like flanked by upstream insertion sequence ISAba1 [41].
bla OXA-40-like β-lactamases
The blaOXA-40- like β-lactamases were the second group of OXA-type β-lactamases to be identified in A. baumanniii solates from the outbreak in Spain. These enzymes can hydrolyze penicillins but show weak hydrolytic properties against cephalosporins and carbapenems, such as imipenam and ceftazidime. These enzymes are resistant to inhibitors such as tazobactam, sulbactam clavulanic acid and NaCl. blaOXA-40-like enzyme (initially called blaOXA-24-like) was isolated from A. baumannii strains, which showed high MIC levels of carbapenem in a study conducted in Spain and amino acids similarity between blaOXA-40-like and two others enzymes bla OXA-51/69 and bla OXA-23-like enzymes was 63% and 60% respectively. Moreover, the group includes bla OXA-24, bla OXA-25, bla OXA-26, bla OXA-72, bla OXA-139 and bla OXA-160 genes. The bla OXA-25 and bla OXA-26 genes were isolated from Spain and Belgium. Another study from Thailand, it originally identified bla OXA-72 gene from CR-Ab strains [37, 42,43,44,45].
Although the poor hydrolyze of the enzyme against carbapenem, The MIC > 128 mg/L for imipenem was shown in isolates expressed blaOXA-40-like [46]. After introducing blaOXA-40-like gene to a sensitive A. baumannii strains, a low to moderate resistance was reported to imipenem. This resistance mostly belongs to the over-expression of the efflux pump from the AdeABC, suggesting involvement of other high-standard phenotype mechanisms [47, 48].
bla OXA-51-like β-lactamases
blaOXA-51 β-lactamase is reported from carbapenem resistance A. baumannii strains worldwide and was initially identified in A. baumannii isolates from Argentina isolated in 1996. Since then, many of these enzymes have been identified. blaOXA-51-like β-lactamases share amino acid similarity about 56% with blaOXA-23-like enzymes, 61–62% homolog with blaOXA-40-like enzymes, while they share less than 50% with blaOXA-58-like enzymes [49, 50].
The activity of these enzymes can be blocked effectively with clavulanic acid, tazobactam or NaCl [51]. Currently, ninety-five enzymes are identified belong to the bla OXA-51-like enzymes. The blaOXA-51 genes are typically non-transferable and encoded by chromosomal DNA (34). Nevertheless, the 2009 research showed that blaOXA-51-like gene could be transferred to other organisms by plasmid. The transformation of a bla OXA-51-like and blaOXA-87, pseudo-gene to DH5α E. coli showed the probability of transmission between plasmids and chromosomal DNA. The blaOXA-51-like enzymes were first identified in other Acinetobacter species (A. nosocomialis) from Taiwan, which are an intrinsic carbapenemase genes in A. baumannii. Thus, this suggests the spread of these resistance genes among baumannii- calcoaceticus complex [52] and it was found that the blaOXA-51 and blaOXA-69 GC contact of these genes were comparable to GC content of the A. baumannii, which suggested they are native to Acinetobacter spp. [53].
A kinetic study showed a low enzymatic activity of both blaOXA-51 and blaOXA-69 against coloxacillin and oxacillin,which is shown among carbapenem-hydrolyzing oxacillinases, This will challenge any attempts to study the hydrolysis activity of them. It has been proposed that bla OXA-51-like expression level is weak but when insertion sequence (IS) elements such as ISAba1 are located upstream of blaOXA genes, high level of resistance occurs. The insertion sequence elements provide a stronger promoter for these genes [54].
A large group of enzymes around the blaOXA-66-like enzymes are recorded in A. baumannii isolates, which involved the common European clone 2. However, enzymes belong to clone 1 in Europe are detected around the blaOXA-69 enzyme and the blaOXA-71 enzyme is related to clone 3 [55]. The most common enzymes in South America and Asia are typically those blaOXA-66-like clusters, while in Eastern Europe there is a common presence of cluster enzymes of blaOXA-69. The European clone 3-associated blaOXA-71 was widely detected in isolates from the Iberian Peninsula [55].
bla OXA-58-like β-lactamase
The gene related to blaOXA-58-like enzymes was initially identified in carbapenem-resistant A. baumannii strain in Toulouse, France. The hydrolysis mechanism of these enzymes was against penicillin, imipenem, and oxacillin, while cannot hydrolysis expended- spectrum cephalosporins. The gene of blaOXA-58-like was found on a non-transferable, 30 k plasmid. When this plasmid transformed to stranded strain of A.baumannii showed reduced carbapenem sensitivity [56]. The gene blaOXA-58-like has only been found in Acinetobacter spp. blaOXA-58-like enzyme showed identical characteristics to other types of blaOXA enzymes. However, hydrolysis of imipenem occurs twice as that of the blaOXA-40-like enzyme. The activity of the enzyme against penicillin or imipenem is poor, meropenem very weak, and cerebrospinomas and cefpiromas have little activity but ceftazidime or celotaxime is not active [54]. Furthermore, insertion strong promoters upstream of these genes cause an increase in synergistic expression leading to high level-carbapenem resistance [54].
The blaOXA-58-like enzymes are a weakly related to other oxacillinases and showed less than 50% amino acid similarity. For some Acinetobacter species, blaOXA-58-like pseudo-enzymes are found to be carried by plasmids; however, the chromosomal location is also described [57]. blaOXA-58-like isolates have been recorded from Europe, South America, North America, Anatolia, Asia and Australia [58, 59]. Besides its dependence on IS-Elements, the degree of expression of blaOXA-58-like genes can be related to the copy number of the gene as shown in an Italian clone [60] and the prevalence of carbapenem resistance was reported in A. genospecies, A. pittii and A. nosocomialis isolates [61, 62]. In Acinetobacter species, other types of the bla OXA-58 are bla OXA-96, bla OXA-97 and bla OXA-164 genes [37, 63].
Biocides resistance
Resistance to antimicrobial and biocides, including antiseptic and disinfectant substances may cause the spread of multidrug resistant strains, which lead to untreatable outbreak incidences in hospitals. The relation between the increase in antimicrobial resistance and disinfectants reducing activity has been reported in some bacteria such as Proteus, P. aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), providencia species, vancomycin-resistant Enterococci and Serrasia marcessens. The same correlation was suggested for A.baumannii as in vitro study showed that antiseptics and disinfectants (70% ethyl alcohol, chlorhexidine, povidone-iodine, didecyldimethylammonium chloride) are used effectively and efficiently among 81 strains against their prevalence. However, using diluted Povidone-iodine (1/3) increased the resistance of bacteria by 18.5% (64). Although antiseptic resistance has not been shown to play an important role in the spread of MDR-Ab outbreaks, un-followed recommended protocols can result in lower concentrations or short-term disinfectant exposure may lead to cross-contamination in hospital settings [64].
Virulence and pathogenicity of A. baumannii
Despite extensive research supporting the A. baumannii as an emerging pathogen, little is still known about eminent factors affecting the epidemic, the virulence and pathogenicity of this bacterium. Nevertheless, there are remarkable pathways for attachment of A. baumannii strains to epithelial cells, superficial anti-phagocytic components and production enzymes, and toxins [65].
A-genetic comparative research between non-pathogenic A. baylyi and A. baumannii has identified the potential genes for the virulence in A. baumannii. These genes play an important role in quorum sensing, regulation of iron and pilli synthesis. A study has shown that type VI of secretion systems [T6SS] stimulates the immune response in eukaryotic cells, which suggested that it might contribute in the interactions between bacteria and host cells in Acinetobacter spp. [65].
Colonization of A. baumannii on human skin and inert surfaces is responsible for infection, disease propagation and persistence of bacteria in the environment. A. baumannii attachment to human bronchial epithelial cells has been demonstrated to be more effective than IC1 strains, but there has been no substantial association between infection-related strains and cell proportion [66].
A. baumannii can invade eukaryotic cells and cause apoptosis following attachment and colonization. An important virulence factor in A. baumannii is outer membrane protein (OmpA), which enhances cell death via mitochondrial and nuclear targeting [67]. In addition, pure OMP38 induces apoptosis of human monocytes and epithelial cells. Apoptosis of epithelial cells may reduce the mucosal surface and may provide a pathway for deep tissue infection by bacteria or in their products [68].
Furthermore, the major virulence factors for Gram-Negative bacteria are capsular polysaccharide (K), glycoproteins and lipoploysaccharide contained O antigen (LpS). Surface polysaccharides have an essential role in bacterial motility and serve as antibacterial barriers. In A. baumannii, the antigen K1 was indicated as required for growth in human ascites fluid, resistance to human serum killing and the mouse model of soft tissue infection [65, 69].
A study of the genome of A. baumannii showed that it lacks ligase-encoding gene waaL and this gene is needed to add O-antigen to the glucose group of LPS, composition of the external leaflet of outer membrane Gram-Negative bacteria [67]. A waaL-like gene found in the genomes of A. baumannii was suggested to be prepaciated in protein glycosylation but not O-antigen linkage [65]. However, there is a second waaaL gene in the A. baumannii was found in some strains, which thought to be required in O-antigen linkage as it does not contain a domain for protein glycosylation [69, 70]. In contrast to previous study, recent comparative genomics of A. baumannii in brazil has suggested no waaL was identified in the strains using blastp and Pfam for gene identification, and Artemis compare gene arrangement [71].
In 2010, Russo et al. identified two predicated genes involved in capsule polymerization and assembly using random transposon (Tn) mutants derived from the A. baumannii strain AB307-0294. These two genes consist of ptks (tyrosine kinase encoding) required to polymerize the capsule and epsA (encoding polysaccharide external membrane protein (EpsA). Acinetobacter baumannii AB307-0294 K1 capsule was associated with human ascetic fluid development, serum and complementary system, as well as mouse infection. Although the absence of ptk or epsA contributes to in vivo strains killing, both enzymes are possible candidates for the drug targets [69, 77, 78]. The variations between A. baumannii strains in the mitogenic activity of LPS are primarily due to O antigens and lipid A differences. LPS of A. baumannii leads to death in mice, pyrogenic effects in rabbits and triggers complement neutralization. Another factor increases the severity of the diseases during sepsis of A. baumannii is Endotoxin secretion. A study showed that Acinetobacter baumannii and Acinetobacter genomospecies nine endotoxins can induce inflammatory signals within human monocytes and Toll-like receptors (TLR2 and TLR4) dependent responses. Despite the ability of Acinetobacter spp to attached to human cells, the stimulation of inflammatory response of these bacteria is weak, which assists in the spread of bacteria and overcome the immune system defence, as defined previously for Haemophilus influenzae [72,73,74,75].
Aceintobacter baumannii quorum sensing
Quorum sensing (QS) is a stimulant mechanism of the autoinducer-receptor. actually, forms cell-to-cell bacterial contact through the production of a stimulating agent. Different forms of QS systems can be employed with Gram-Negaive bacteria:
-
(I)
Acyl homoserine lactones (AHLs), called AI-1 (AutoInducer-1), are generated mainly in bacteria. Every molecule consists of a lactone ring and an aliphatic chain with different lengths and characterization such as Pseudomonas spp., Acinetobacter spp., Burkholderia spp.
-
(II)
Furanosyl-Borate Diester, Autoinducer-2 (AI-2), presents in both Gram-Negaive and Gram-Positive bacteria for example Vibrio spp. and Pectobacterium spp.
-
(III)
Epinephrine and norepinephrine, known as Autoinducer-3 (AI-3) are commonly characterized in human opportunistic pathogens such as Enterobacter spp., Escherichia spp., Klebsiella spp. and Salmonella spp. In addition, other molecules have also been found such as (IV) fatty acids DSF in Xanthomonas spp., (V) Palmitic acid methyl ester (PAME) in Ralstonia solanacearum, (VI) α-Hydroxyketone in Legionella spp. and Vibrio spp. or (VII) 2‐heptyl‐3‐hydroxy‐4‐quinolone (PQS) Pseudomonas aeruginosa [76,77,78,79,80,81,82,83,84,85].
Most of A. baumannii strains generate more than one molecule of AHL. The communications between cells aim to coincide with the expression of target genes and organize the biological functions of the population. This mechanism has an important role related to virulence factors expression, plasmid transformation, motility, antimicrobial compounds secretion, bacterial attachment, formation of biofilm and sporulation. None of the AHL signals may be unique to a particular Acinetobacter species. Furthermore, QS signals were not homogeneously distributed, making it difficult to differentiate between virulent and non-virulent strains. The abaI and abaR genes were not acquired horizontally from Halothiobacillus neapolitanus into strains of A. baumannii [82,83,84,85,86,87]. Combating QS is called quorum quenching (QQ), which can occur in different methods, like, LuxR homolog proteins inhibition, inhibition of the enzyme activity, destruction of QS signals, the destruction of AHL efflux protein and QS antagonists [78, 80, 81, 85, 88].
A. baumannii and host interactions
Interactions between pathogens and host may be controlled by different factors, such as virulence, bacterial burden, and host factors beside it plays a significant role in development of infections causing serious effects on the host. It has found that cluster of 28 genes are unique to A. baumannii in comparison to Acinetobacter 17978 strain, non-pathogenic A. baylyi species. 16 genes of these clusters play a major role in the virulence [89]. 6 genes are present in csu polycistronic operon csuA/B, csuA, csuB, csuC, csuD, and csuE and Some of them are homologs to encoding chaperone-related genes, which participate in the accumulation of germ cell in Gram-Negative bacteria [87, 88]. It is probably; formation of biofilm plays an important role in the interactions of A. baumannii with its host besides biofilm contribution to infections associated with medical-devices [65, 90].
The biofilm formation in A. baumannii has been demonstrated that it is phenotypically related to the production exo-polysaccharide and pilus structures as host defence-protected bacteria. Within structures similar to the Pilli, the bone marrow of human bronchial cells and erythrocytes is also shown. Through these surface structures, A. baumannii strains attach to the host cells [78, 84, 91, 92]. The role of innate immunity against A. baumannii infections has been addressed via Toll-like receptor (TLR) signaling. In mouse model with pneumonia, mice without TLR4 showed elevated in bacteria number resulting in bacteremia, cytokine / chemokine disorders and slow lung inflammation in comparison group in the study. The LPS play a major role in triggering immune response and this result was further showed by decreased effects of A. baumannii in mice with a CD14 deficiency, which is an effective LPS receptor for TLR4 attachment [93].
These results confirmed by studies on mouse and human cells models demonstrating that TLR2 played important role in the signaling pathway and A. baumannii LPS (endotoxin) induction through stimulation of inflammatory cytokines, TNF-α (tumor necrosis factor alpha), interleucine 8 close to stimulating effects induced by Escherichia coli LPS [74]. These studies suggested that a strong inflammatory response during infection with the A. baumannii could be related to endodotoxin. Antibodies, which bind to iron suppressor OMPs and the O antigen in the LPS also produce a humoral immune response to A. baumannii infection. Monoclonal antibodies derived from the mouse against A. baumannii OMPs in CDM-Fe medium showed opsonization, antimicrobial activity and iron uptake inhibition [94].
Biofilm formation
Biofilm formation on the surfaces of medical devices is considered a major source of infections in hospitals around the world. Agents like bacterial hydrophobicity are mediated the non-specific attachment [95, 96]. The interaction with the host cells, mostly through specific receptors is the initial stage in the colonization or infection cycle. Bacteria are now developing micro-colonies contributing to the creation of a cumulative structure network known as biofilm. Biofilms are formed on living and no living surfaces and their matrix is composed of carbohydrates, nucleic acids, proteins and other macromolecules. Biofilm protects bacteria from the damage of the environment as host responses, antibiotics, cleansers, and disinfectants. Therefore, biofilms contribute to prolonged and more severe bacterial effects. In addition, different factors such as bacterial structures, nutrients, surface components, QS, and bacterial hidden molecules affect biofilms [97]. A variety of regulatory networks are less known including a two-component regulatory system (TCS) and environmental reactive transcription regulatory systems, which regulate the biofilm genes expression. The formation of biofilm in A. baumanni depends on biosynthesis and assembly of pili, which involved in surfaces attachment and coded via the csuA/BABCDE chaperon-usher system [95]. The result showed that inactivation of the csuC and csuE ORFs that locate within the csuA/BABCDE operon leading to non-piliated cells and abolishes cell attachment. This suggested that the CsuA/BABCDE system is involved in early stage of biofilm formation [95]. Another TCS regulates the expression of csuA/BABCDE operon involved a sensor kinase encoded by the bfmS gene and a response regulator encoded by the bfmR [97]. The bfmR suppression causes the lack of the csu operon expression level, which affects the expression of Pilli and the formation of biofilm. Furthermore, attachment between the host cell receptors and Pilli triggers the induction of inflammatory mediators such as chemokines and cytokines. A CsuA/BABCDE independent short pilus is produced, which assists in bacterial attachment to biotic surfaces such as human respiratory cells [87]. Moreover, another TCS known as LuxI /LuxR QS is involved in this process. Biofilm-producing A. baumannii strains showed higher survival rate than strains do not form biofilm [98]. A. baumannii strains showed a survival rate of about 10 to 13 days on undisturbed dry surfaces in the ICU settings in comparison to other Gram-Negative bacteria. Furthermore, these strains are able to survive on the hospital bed rails and in wet environments [99]. Polysaccharide layer has been observed outside the surface of biofilm-producing strains but not among those non-biofilm producers using electron microscopy. In addition, LPS layer is used by bacteria to protect from alterations in environmental humidity and assists in antibiotics resistance [100]. It was observed where a mutation occurs in the abaI gene that encodes the auto-inducer synthase gene, which leads to reduce the ability to form biofilm [101].
Iron regulation
The iron deficiency increases during A. baumannii invasion process but these bacteria can acquire ferric ions under iron-limited conditions via siderophore [102]. Therefore, multiple sidephores expression is common between pathogenic bacteria and associated with their virulence. Siderophores synthesised by A. baumannii are relatively low-molecular-weight agents and able to convert polymeric ferric oxy-hydroxides to soluble iron chelates under iron stress condition. [6, 19]. Ten distinct siderophores are elaborated by A. baumannii and encoded by three different loci. The major sidephore for A. baumannii is acinetobactin, which is shown as the only siderophore associated with the virulence [103]. This is a recent study 2020 (105).
Bacteriophage as therapeutic for MDR A. baumannii
An increase in resistance to available antibiotics causes an argent alarm to investigate alterative therapeutic options. The significant clinical interest has focused on bacteriophage therapy. Bacteriophages are natural antibiotics that are able to work against Gram-Positive and Gram-Negative bacteria [104, 105]. Phages can be isolated rapidly, because of their ubiquitous nature and they are abundant in every ecological niche, which reduces their development costs compared to antibiotics. Bacteriophages are viruses restricted to bacteria and found in different environments and they can be isolated from water, soil, sewage, hospitals, hot springs, and faecal material, and also humans, soil, and animals' gastrointestinal tracts [106]. It seems that the phage therapy with no detectable side effects and high specificity to pathogen they infect on species or strain level, which reduce the effect on microflora inside human gut compared to antibiotics [107]. Another advantage of phages can spread fast in all the body and organs such as the brain, prostate gland, and bones, which usually the antibiotics do not reach. In addition, the presence of phage hosts assists in successful treatment of infections caused by these hosts. Furthermore, no cross-resistance can be developed against phage like antibiotics, which bacteria can develop resistance mechanism easily. Thus, bacteriophage therapy can provide an effective treatment against MDR, XDR, and PDR bacteria [108,109,110]. Using phage therapy has been studied to treat bacterial infections for about 100 years. These phages attack and kill their bacterial hosts by lysis without attacking the human cells. In addition, bacteriophages are specific to bacteria and bind to a specific receptor on bacterial cell wall followed by injection of their genetic material to lysis the cells [111]. Despite the advantages of phage therapy mentioned above and many clinical trials to use it, some drawbacks are mentioned in the literature, we enumerate a few of them: specificity of phage can be considered as a disadvantage as before any treatment need to specify the etiological microorganism causing infection. Another problem is the ability of these phages to transfer DNA between bacteria, which can transfer pathogenicity determinants and virulence factors from one bacterium to another. This can lead to develop a more resistant microorganism or even a new one. In addition, bacteriophages are viruses and can be seen as opportunistic pathogens by the patient immune system, which leads to eliminating them especially in case of prolonged or repeated applications. Another concern is the ability of the bacterial host to develop resistance from mutation and selection or by temperate phage acquisition reviewed by [109].
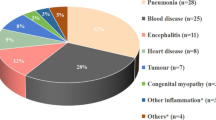
Many recent attempts have been started to address bacteriophage therapy against MDR A baumannii Fig. 1. A recent report has shown that an older patient with MDR A baumannii was treated with bacteriophages. These patients suffered assault, subdural hematoma, and traumatic brain injury and led to complicated consequences. A baumannii isolated from this patient was resistant to all antibiotics and the antibiotics combination treatment was without improvement. The patient was in the ICU during administration of the phage and this statue was frequently monitored for neurologic or hemodynamic changes. The first dose was without any complications expected after two hours he suffered hypotensive but did not require vasopressors. Even infections were started to heal, the patient was unresponsive [112]. Therefore, they did not have the complete set of data to monitor phage efficacy beyond the patient’s clinical status and they cannot conclude the lack or failure of phage therapy in this patient, which requires other trails in this field.
Another study has isolated a novel A. baumannii phage Βϕ-R2096 from sewage water to lysis carbapenem-resistant A. baumannii isolated from a patient in the university hospital in South Korea. They used animal model Galleria mellonella larvae and a mouse model of acute pneumonia to study the efficiency of A. baumannii phage Bϕ-R2096.They showed that the phage was effective in virto and showed a significant improvement in survival rates of Galleria mellonella larvae without any toxicity. However, survival rates were higher in the mouse infection model [113].
Very recent study in vitro, Altamirano et al. showed that phage therapy was effective in carbapenem-resistant A. baumannii. The isolated mutants caused the strains to be resistant to the phage and they found these mutants in K locus, which is involved in capsular polysaccharide. This polysaccharide was hypothesized that phages used them as receptors. Thus, loss or any modification of these receptors causes a lack of phage adsorption and subsequent phage-resistance in A. baumannii. Furthermore, they found that these mutants showed a decrease in biofilm formation antibiotic resistance. This study suggested that these bacteriophages cannot be used for their lytic activity only but the combination between understanding of phage receptors and bacterial resistance mechanism provides the best knowledge of the potential synergy effect of both phage and antimicrobial agents [91, 114]. Therefore, future attention should be focused on bacteriophage therapy especially for A. baumannii with MDR resistance, which causes many epidemics in hospitals. In Iraq, these bacteria showed resistance to all antibiotics in use and cause an endemic inside Baghdad hospitals (unpublished data). Thus, any better available treatment options against MDR bacteria will save the lives of many patients attending hospitals worldwide.
Conclusion
Acinetobacter baumannii has brought attention as a public health problem and its infections prevalence and outbreaks due to extensive antibiotic resistance. Few known antibiotics are effective to treat infections caused by these bacteria. The high mortality rate caused by multi drug resistance bacteria is a serious problem challenge physician and healthcare workers to eliminate these infections either originated from the hospitals or the community settings. Most of infections caused by these emerging strains are among patients in ICU who have developed resistance to antimicrobials over the past three decades including resistance to carbapenems, which areused as the last-line antibiotics for the treatment of infections caused by Gram-Negative bacteria. Genetic elements such as transposons, plasmids or integrons, and carrying resistant cassettes have a major role in acquisition of antimicrobial resistance and dissemination of MDR strains. An increase in the prevalence of carbapenem-resistant A. baumannii worldwide has become a critical problem and the development of pathogenic MDR-Ab will make infections caused by these bacteria hard to be treated and Col-R Ab has been recorded. Attachment, biofilm formation, secretion of toxins beside weak induction of inflammatory responses are mechanisms associated with A. baumannii pathogenic strains. Therefore, to overcome the problem associated with these infections, investigation of the pathogenesis and mechanisms of antibiotic resistance of A. baumannii is important. Furthermore, new strategies of treatments like phage therapy are required to overcome infections caused by these resistant bacteria.
References
van Looveren M, Goossens H, ARPAC Steering Group (2004) Antimicrobial resistance of Acinetobacterspp Europe. Clin Microbiol Infect 10(8):684–704
Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10:539
Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodríguez-Valera F (2005) Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43(9):4382–4390
Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5(12):939–951
Karmostaji A, Peerayeh SN, Salmanian AH (2013) Distribution of OXA-type class D β-lactamase genes among nosocomial multi drug resistant Acinetobacter baumannii isolated in Tehran hospitals. Jundishapur J Microbiol 6(5):45
Antunes L, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71(3):292–301
Dehbalaei MA, Najar-Peerayeh S, Taherikalani M, Behmanesh M (2017) Clinical isolates of Acinetobacter baumannii from tehran hospitals: pulsed-field gel electrophoresis characterization, clonal lineages, antibiotic susceptibility, and biofilm-forming ability. Jundishapur J Microbiol 10(7):56
Cisneros J, Rodriguez-Bano J (2002) Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect 8(11):687–693
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21(3):538–582
Al-Kadmy IMS, Ali ANM, Salman IMA, Khazaal SS (2018) Molecular characterization of Acinetobacter baumannii isolated from Iraqi hospital environment. New Microbes New Infect 21:51–57
Garnacho-Montero J, Ortiz-Leyba C, Fernández-Hinojosa E, Aldabó-Pallás T, Cayuela A, Marquez-Vácaro JA, Garcia-Curiel A, Jiménez-Jiménez FJ (2005) Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 31(5):649–655
Khazaal SS, Al-Saryi N, Ibrahim SA (2020) Immunomodulation by Acinetobacter baumannii of endotracheal tube biofilm in ventilator-associated pneumonia. Meta Gene 13:100672
Lee H-Y, Chen C-L, Wu S-R, Huang C-W, Chiu C-H (2014) Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med 42(5):1081–1088
Chopra T, Marchaim D, Awali RA, Krishna A, Johnson P, Tansek R, Chaudary K, Lephart P, Slim J, Hothi J, Ahmed H, Pogue JM, Zhao JJ, Kaye KS (2013) Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother 57(12):6270–6275
Khazaal SS, Al-Kadmy IM, Aziz SN (2020) Mechanism of pathogenesis in multidrug resistant Acinetobacter baumannii isolated from intensive care unit. Gene Rep 18:100557
Hakemi Vala M, Hallajzadeh M, Hashemi A, Goudarzi H, Tarhani M, Sattarzadeh Tabrizi M, Bazmi F (2014) Detection of Ambler class A, B and D ß-lactamases among Pseudomonas aeruginosa and Acinetobacter baumannii clinical isolates from burn patients. Ann Burns Fire Disasters 27(1):8–13
Kareem SM, Al-Kadmy IM, Kazaal SS, Ali ANM, Aziz SN, Makharita RR, Algammal AM, Al-Rejaie S, Behl T, Batiha GES, El-Mokhtar MA (2021) Detection of gyra and parc mutations and prevalence of plasmid-mediated quinolone resistance genes in klebsiella pneumoniae. Infect Drug Resist 14:555
Fernandes R, Amador P, Prudêncio C (2013) β-lactams: chemical structure, mode of action and mechanisms of resistance. Rev Med Microbiol 24(1):7–17
Jean S-S, Hsueh P-R (2011) High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents 37(4):291–295
Velkov T, Dai C, Ciccotosto GD, Cappai R, Hoyer D, Li J (2017) Polymyxins for CNS infections: pharmacology and neurotoxicity. Pharmacol Ther 6(5):e8219
Zhang W, Aurosree B, Gopalakrishnan B, Balada-Llasat J-M, Pancholi V, Pancholi P (2017) The role of Lpxa/c/d and pmra/b gene systems in colistin-resistant clinical strains of Acinetobacter baumannii. Front Lab Med 1(2):86–91
Al-Kadmy IMS, Ibrahim SA, Al-Saryi N, Aziz SN, Besinis A, Hetta HF (2020) Prevalence of genes involved in colistin resistance in Acinetobacter baumannii: first report from Iraq. Microbical Drug Resist 26(6):616–622
Hussein NH, Al-Kadmy IM, Taha BM, Hussein JD (2021) Mobilized colistin resistance (mcr) genes from 1 to 10: a comprehensive review. Mol Biol Rep 48(3):1–11
Hujer AM, Higgins PG, Rudin SD, Buser GL, Marshall SH, Xanthopoulou K, Seifert H, Rojas LJ, Domitrovic TN, Cassidy PM, Cunningham MC, Vega R, Furuno JP, Pfeiffer CD, Beldavs ZG, Wright MS, Jacobs MR, Adams MD, Bonomo RA (2017) Nosocomial Outbreak of extensively drug-resistant Acinetobacter baumannii isolates containing blaOXA-237 carried on a plasmid. Antimicrob Agents Chemother 61(11):e00797-e817
Music MS, Hrenovic J, Goic-Barisic I, Hunjak B, Skoric D, Ivankovic T (2017) Emission of extensively-drug-resistant Acinetobacter baumannii from hospital settings to the natural environment. J Hospit Infect 96(4):323–327
Wilharm G, Skiebe E, Higgins PG, Poppel MT, Blaschke U, Leser S, Heider C, Heindorf M, Brauner P, Jäckel U, Böhland K, Cuny C, Łopińska A, Kaminski P, Kasprzak M, Bochenski M, Ciebiera O, Tobółka M, Żołnierowicz KM, Siekiera J, Seifert H, Gagné S, Salcedo SP, Kaatz M, Layer F, Bender JK, Fuchs S, Semmler T, Pfeifer Y, Jerzak L (2017) Relatedness of wildlife and livestock avian isolates of the nosocomial pathogen Acinetobacter baumannii to lineages spread in hospitals worldwide. Environ Microbiol 19(10):4349–4364
Kareem SM, Al-Kadmy IM, Al-Kaabi MH, Aziz SN, Ahmad M (2017) Acinetobacter baumannii virulence is enhanced by the combined presence of virulence factors genes phospholipase C (plcN) and elastase (lasB). Microb Pathog 110:568–572
Bogaerts P, Naas T, Wybo I, Bauraing C, Soetens O, Piérard D, Nordmann P, Glupczynski Y (2006) Outbreak of infection by carbapenem-resistant Acinetobacter baumannii producing the carbapenemase OXA-58 in Belgium. J Clin Microbiol 44(11):4189–4192
Saranathan R, Vasanth V, Vasanth T, Shabareesh PRV, Shashikala P, Devi CS, Kalaivani R, Asir J, Sudhakar P, Prashanth K (2015) Emergence of carbapenem non-susceptible multidrug resistant Acinetobacter baumannii strains of clonal complexes 103B and 92B harboring OXA type carbapenemases and metallo-β-lactamases in Southern India. Microbiol Immunol 59(5):277–284
Corvec S, Poirel L, Naas T, Drugeon H, Nordmann P (2007) Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob Agents Chemother 51(4):1530–1533
Figueiredo S, Poirel L, Seifert H, Mugnier P, Benhamou D, Nordmann P (2010) OXA-134, a naturally occurring carbapenem-hydrolyzing class D β-lactamase from Acinetobacter lwoffii. Antimicrob Agents Chemother 54(12):5372–5375
Paton R, Miles R, Hood J, Amyes S (1993) ARI 1: β-lactamase-mediated imipenem resistance in Acinetobacter baumannii. Int J Antimicrob Agents 2(2):81–87
Afzal-Shah M, Woodford N, Livermore DM (2001) Characterization of Oxa-25, oxa-26, and oxa-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 45(2):583–588
Evans BA, Amyes SG (2014) Oxa β-lactamases. Clin Microbiol Rev 27(2):241–263
Donald HM, Scaife W, Amyes SG, Young HK (2000) Sequence analysis of ARI-1, a novel OXA beta-lactamase, responsible for imipenem resistance in Acinetobacter Baumannii 6B92. Antimicrob Agents Chemother 44:196–199
Héritier C, Poirel L, Lambert T, Nordmann P (2005) Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 49(8):3198–3202
Evans BA, Sebastian GB (2014) Amyes. OXA β-lactamases. Clin Microbiol Rev 27(2):241–263
Poirel L, Naas T, Nordmann P (2010) Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother 54(1):24–38
Villegas MV, Kattan JN, Correa A, Lolans K, Guzman AM, Woodford N, Livermore D, Quinn JP (2007) Dissemination of Acinetobacter baumannii clones with OXA-23 carbapenemase in Colombian hospitals. Antimicrob Agents Chemother 51(6):2001–2004
Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, Yu Y, Li L (2010) Wide dissemination of Oxa-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother 65(4):644–650
Wang X, Zong Z, Lü X (2011) Tn2008 is a major vehicle carrying bla oxa-23 in Acinetobacter baumannii from China. Diagn Microbiol Infect Dis 69(2):218–222
Walther-Rasmussen J, Høiby N (2006) Oxa-type carbapenemases. J Antimicrob Chemother 57(3):373–383
Poirel L, Nordmann P (2006) Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12(9):826–836
Povilonis J, Seputiene V, Krasauskas R, Juskaite R, Miskinyte M, Suziedelis K, Suziedeliene E (2013) Spread of carbapenem-resistant Acinetobacter baumannii carrying a plasmid with two genes encoding OXA-72 carbapenemase in Lithuanian hospitals. J Antimicrob Chemother 68:1000–1006
Chen Y, Yang Y, Liu L, Qiu G, Han X, Tian S, Zhao J, Chen F, Grundmann H, Li H, Sun J, Han L (2018) High prevalence and clonal dissemination of Oxa-72-producing acinetobacter baumannii in a Chinese hospital: a cross sectional study. BMC Infect Dis 18(1):491
Heritier C, Poirel L, Aubert D, Nordmann P (2003) Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob Agents Chemother 47(1):268–273
Héritier C, Poirel L, Aubert D, Nordmann P (2003) Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob Agents Chemother 47(1):268–273
Coyne S, Courvalin P, Périchon B (2011) Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother 55(3):947–953
Brown S, Amyes S (2005) The sequences of seven class D β-lactamases isolated from carbapenem-resistant Acinetobacter baumannii from four continents. Clin Microbiol Infect 11(4):326–329
Poirel L, Marqué S, Héritier C, Segonds C, Chabanon G, Nordmann P (2004) OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 49(1):202–208
Bush K, Jacoby GA (2010) Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 54(3):969–976
Lee Y-T, Turton J, Chen T-L, Wu RC-C, Chang W-C, Fung C-P, Chen CP, Cho WL, Huang LY, Siu LK (2009) First identification of bla OXA-51-like in non-baumannii Acinetobacter spp. J Chemother 21(5):514–520
Heritier C, Poirel L, Fournier PE, Claverie JM, Raoult D, Nordmann P (2005) Characterization of the naturally occurring oxacillinase of Acinetobacter baumannii. Antimicrob Agents Chemother 49(10):4174–4179
Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL (2006) The role of IS Aba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett 258(1):72–77
Evans BA, Hamouda A, Towner KJ, Amyes SG (2008) Oxa-51-like β-lactamases and their association with particular epidemic lineages of Acinetobacter baumannii. Clin Microbiol Infect 14(3):268–275
Poirel L, Marqué S, Héritier C, Segonds C, Chabanon G, Nordmann P (2005) OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 49(1):202–208
Hammoudi HD, Ayoub MC (2020) The current burden of carbapenemases: review of significant properties and dissemination among gram-negative bacteria. Antibiotics 9(4):186
Rodríguez CH, Yarhui NB, Nastro M, Quezada TN, Cañarte GC, Ventura RM, Ugarte Cuba T, Valenzuela N, Roach F, Mota MI, Burger N, Velazquez Aguayo G, Ortellado-Canese J, Bruni G, Pandolfo C, Bastyas N, Famiglietti A (2016) Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in South America. J Med Microbiol 65(10):1088–1091
Bogaerts P, Naas T, El Garch F, Cuzon G, Deplano A, Delaire T, Huang TD, Lissoir B, Nordmann P, Glupczynski Y (2010) GES extended-spectrum β-lactamases in Acinetobacter baumannii isolates in Belgium. Antimicrob Agents Chemother 54(11):4872–4878
Bertini A, Poirel L, Bernabeu S, Fortini D, Villa L, Nordmann P, Carattoli A (2007) Multicopy bla OXA-58 gene as a source of high-level resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 51(7):2324–2328
Evans B, Hamouda A, Towner K, Amyes S (2010) Novel genetic context of multiple bla OXA-58 genes in Acinetobacter genospecies 3. J Antimicrob Chemother 65(8):1586–1588
Singkham-In U, Chatsuwan T (2018) Mechanisms of carbapenem resistance in Acinetobacter pittii and Acinetobacter nosocomialis isolates from Thailand. J Med Microbiol 67(12):1667–1672
Lanjri S, Uwingabiye J, Frikh M, Abdellatifi L, Kasouati J, Maleb A, Bait A, Lemnouer A, Elouennass M (2017) In vitro evaluation of the susceptibility of Acinetobacter baumannii isolates to antiseptics and disinfectants: comparison between clinical and environmental isolates. Antimicrob Resist Infect Control 6(36):1–7
Kouyama Y, Harada S, Ishii Y, Saga T, Yoshizumi A, Tateda K, Yamaguchi K (2012) Molecular characterization of carbapenem-non-susceptible Acinetobacter spp in Japan: predominance of multidrug-resistant Acinetobacter baumannii clonal complex and IMP-type metallo-β-lactamase-producing non-baumannii Acinetobacter species. J Infect Chemother 18(4):522–528
Harding CM, Hennon SW, Feldman MF (2018) Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 16(2):91–102
Giannouli M, Antunes LC, Marchetti V, Triassi M, Visca P, Zarrilli R (2013) Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes st25 and st78. BMC Infect Dis 13:282
Smani Y, Fàbrega A, Roca I, Sánchez-Encinales V, Vila J, Pachón J (2014) Role of Ompa in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob Agents Chemother 58(3):1806–1808
Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, Kim SA, Lee SK, Lee JC (2005) Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol 7(8):1127–1138
Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, Schultz LW, Umland TC, Campagnari AA (2010) The K1 capsular polysaccharide of Acinetobacter baumannii strain 307–0294 is a major virulence factor. Infect Immun 78(9):3993–4000
Eijkelkamp BA, Stroeher UH, Hassan KA, Paulsen IT, Brown MH (2014) Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics 15(1):1020
Leal NC, Campos TL, Rezende AM, Docena C, Mendes-Marques CL, de Sá Cavalcanti FL, Wallau GL, Rocha IV, Cavalcanti CLB, Veras DL, Alves LR, Andrade-Figueiredo M, de Barros MPS, de Almeida AMP, de Morais MMC, Leal-Balbino TC, Xavier DE, de AMelo-Neto OP (2020) Comparative genomics of Acinetobacter baumannii clinical strains from brazil reveals polyclonal dissemination and selective exchange of mobile genetic elements associated with resistance genes. Front Microbiol 11:1176
Moffatt JH, Harper M, Mansell A, Crane B, Fitzsimons TC, Nation RL, Li J, Adler B, Boyce JD (2013) Lipopolysaccharide-deficient Acinetobacter baumannii shows altered signaling through host toll-like receptors and increased susceptibility to the host antimicrobial peptide LL-37. Infect Immun 81(3):684–689
Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD (2010) Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54(12):4971–4977
Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR (2007) Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol 56(Pt 2):165–171
Bosch AATM, Biesbroek G, Trzcinski K, Sanders EAM, Bogaert D (2013) Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 9(1):1003057
Chen X, Schauder S, Potier N, Dorsselaer AV, Pelczer I, Bassler BL, Highson FM (2002) Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415(6871):545–549
Churchill MEA, Chen L (2011) Structural basis of acyl-homoserine lactone dependent signaling. Chem Rev 111(1):68–85
Hetta HF, Al-Kadmy IM, Khazaal SS, Abbas S, Suhail A, El-Mokhtar MA, Abd Ellah NH, Ahmed EA, Abd-Ellatief RB, El-Masry EA, Batiha GES (2021) Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci Rep 11(1):1–11
Ryan RP, An S, Allan JH, McCarthy Y, Dow JM (2015) The DSF family of cell-cell signals: an expanding class of bacterial virulence regulators. PLoS Pathog 11(7):1004986
Cai Z, Yuan Z-H, Zhang H, Pan Y, Wu Y, Tian X-Q, Wang F-F, Li W, Qain W (2017) Fatty acid DSF binds and allosterically activates histidine kinase RpfC of phytopathogenic bacterium Xanthomonas campestris pv. campestris to regulate quorum-sensing and virulence. PLoS Pathog 13(4):1006304
Flavier AB, Clough SJ, Schell MA, Denny TP (1997) Identification of 3- hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol 26(2):251–259
Winans SC (2011) A new family of quorum sensing pheromones synthesized using S-adenosylmethionine and Acyl-CoAs. Mol Microbiol 79:1403–1406
Tiaden A, Hilbi H (2012) α-Hydroxyketone synthesis and sensing by legionella and vibrio. Sensors (Basel) 12(3):2899–2919
Lee J, Zhang L (2015) The hierarchy quorumsensing network in Pseudomonas aeruginosa. Protein Cell 6(1):26–41
Kalia VC (2015) Quorum sensing vs. quorum quenching: a battle with no end in sight. Springer, New Delhi
Chow JY, Yang Y, Tay SB, Chua KL, Yew WS (2014) Disruption of biofilm formation by the human pathogen Acinetobacter baumannii using engineered quorum-quenching lactonases. Antimicrob Agents Chemother 58(3):1802–1805
Bhargava N, Sharma P, Capalash N (2010) Quorum sensing in Acinetobacter: an emerging pathogen. Crit Rev Microbiol 36(4):349–360
Hong K-W, Koh C-L, Sam C-K, Yin W-F, Chan K-G (2012) Quorum quenching revisited—from signal decays to signaling confusion. Sensors 12(4):4661–4696
Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M (2007) New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev 21(5):601–614
Asaad AM, Ansari S, Ajlan SE, Awad SM (2021) Epidemiology of biofilm producing Acinetobacter baumannii nosocomial isolates from a tertiary care hospital in Egypt: a cross-sectional study. Infect Drug Resist 23(14):709–717
Alyousef AA, Al-Kadmy IM (2017) The effect of immune modulation of Streptococcus constellatus SC10 strain upon Acinetobactor baumannii infection. Microb Pathog 111:370–374
Brossard KA, Campagnari AA (2012) The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80(1):228–233
Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N et al (2006) Differential roles of CD14 and toll-like receptors 4and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med 173(1):122–129
Goel VK, Kapil A (2001) Monoclonal antibodies against the iron regulated outer membrane Proteins of Acinetobacter baumannii are bactericidal. BMC Microbiol 1(16):59
Marjani MFA, Ali FS, Authman SH, Kadmy IMA, Amir RMA (2020) Identification of novel 1, 3-oxazole and imidazole-5-one that inhibits bacterial biofilm formation of Acinetobacter baumannii. Gene Rep 20:100782
Berne C, Ducret A, Hardy GG, Brun YV (2015) Adhesins involved in attachment to abiotic surfaces by gram-negative bacteria. Microbiol Spectr 3(4):10
Gaddy JA, Actis LA (2009) Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4(3):273–278
Espinal P, Martí S, Vila J (2012) Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect 80(1):56–60
Catalano M, Quelle LS, Jeric PE, Di Martino A, Maimone SM (1999) Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J Hosp Infect 42(1):27–35
Giles SK, Stroeher UH, Eijkelkamp BA, Brown MH (2015) Identification of genes essential for pellicle formation in Acinetobacter baumannii. BMC Microbiol 15(1):116
Niu C, Clemmer KM, Bonomo RA, Rather PN (2008) Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol 190(9):3386–3392
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71(3):413–451
Sheldon JR, Skaar EP (2020) Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog 16(10):1008995
Lood R, Winer BY, Pelzek AJ et al (2015) Novel phage lysins capable of killing the multidrug resistant Gram-negative bacterium Acinetobacter baumannii in a mouse sepsis model. Antimicrob Agents Chemother 4641:14
Nakonieczna A, Cooper CJ, Gryko R (2015) Bacteriophages and bacteriophage-derived endolysins as potential therapeutics to combat gram-positive spore forming bacteria. J Appl Microbiol 119:620–631
Pereira C, Moreirinha C, Teles L et al (2017) Application of phage therapy during bivalve depuration improves Escherichia coli decontamination. Food Microbiol 61:102–112
Nobrega FL, Costa AR, Kluskens LD, Azeredo J (2015) Revisiting phage therapy: new applications for old resources. Trends Microbiol 23:185–191
Yin S, Huang G, Zhang Y, Jiang B, Yang Z, Dong Z, You B, Yuan Z, Hu F, Zhao Y, Peng Y (2017) Phage abp1 rescues human cells and mice from infection by pan-drug resistant Acinetobacter baumannii. Cell Physiol Biochem 44(6):2337–2345
Wittebole X, Roock DE, Opal SM (2014) A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 5:226–235
Shen GH, Wang JL, Wen FS, Chang KM, Kuo CF, Lin CH, Luo HR, Hung CH (2012) Isolation and characterization of φkm18p, a novel lytic phage with therapeutic potential against extensively drug resistant Acinetobacter baumannii. PLoS ONE 7:e46537
Clokie MR, Millard AD, Letarov AV, Heaphy S (2011) Phages in nature. Bacteriophage 1(1):31–45
LaVergne S, Hamilton T, Biswas B, Kumaraswamy M, Schooley RT, Wooten D (2018) Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis 5(4):064
Jeon J, Park JH, Yong D (2019) Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol 19(1):70
Altamirano FG, Forsyth JH, Patwa R, Kostoulias X, Trim M, Subedi D, Archer S, Morris FC, Oliveira C, Kielty L, Korneev D (2020) Bacteriophages targeting Acinetobacter baumannii capsule induce antimicrobial resensitization. bioRxiv 28:157–161
Acknowledgements
The authors would like to thank Mustansiriyah University (https://uomustansiriyah.edu.iq/)/Baghdad, Iraq for its support to complete this work.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed equally in writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
No need approval.
Research involving human participants
No human sample was used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, S., Al-Saryi, N., Al-Kadmy, I.M.S. et al. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol Biol Rep 48, 6987–6998 (2021). https://doi.org/10.1007/s11033-021-06690-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06690-6