Abstract
Cu6Sn5 intermetallic occurs in the form of differently ordered phases η, η′ and η′′. In solder joints, this intermetallic can undergo changes in composition and the state of order without or while interacting with excess Cu and excess Sn in the system, potentially giving rise to detrimental changes in the mechanical properties of the solder. In order to study such processes in fundamental detail and to get more detailed information about the metastable and stable phase equilibria, model alloys consisting of Cu3Sn + Cu6Sn5 as well as Cu6Sn5 + Sn-rich melt were heat treated. Powder x-ray diffraction and scanning electron microscopy supplemented by electron backscatter diffraction were used to investigate the structural and microstructural changes. It was shown that Sn-poor η can increase its Sn content by Cu3Sn precipitation at grain boundaries or by uptake of Sn from the Sn-rich melt. From the kinetics of the former process at 513 K and the grain size of the η phase, we obtained an interdiffusion coefficient in η of (3 ± 1) × 10−16 m2 s−1. Comparison of this value with literature data implies that this value reflects pure volume (inter)diffusion, while Cu6Sn5 growth at low temperature is typically strongly influenced by grain-boundary diffusion. These investigations also confirm that η′′ forming below a composition-dependent transus temperature gradually enriches in Sn content, confirming that Sn-poor η′′ is metastable against decomposition into Cu3Sn and more Sn-rich η or (at lower temperatures) η′.
Graphic Abstract

Similar content being viewed by others
Introduction
Sn-rich alloy systems are of fundamental importance in understanding the microstructural changes occurring during the course of many soldering processes, in particular lead-free soldering1,2,3. Knowledge of the phase diagram provides the basic fundament for predicting these changes. But, as it turns out again and again, even in many binary systems, knowledge of phase equilibria can be very incomplete. Hence, an improved understanding of relevant phase constitution, including relevant metastable equilibria, will contribute to improving processes like soldering by adapting the time−temperature program and chemical composition of the system. As Cu is one of the most import metallic elements in electronics to be soldered, the Cu−Sn system can be regarded as the most fundamental for understanding most soldering processes.
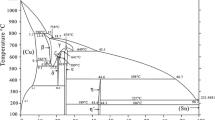
The most common phase diagrams of the Cu−Sn system contain about 45 at.% Sn in two different phases constituting what we refer to as the Cu6Sn5 intermetallic4,5,6: an η high-temperature and an η′ low-temperature phase (compare Fig. 1a, grey lines). The crystal structure of the η phase is of the B81/B82 or NiAs/Ni2In type with a hexagonal unit cell and P63/mmc space group symmetry (compare Fig. 2). Cu(1) and Sn atoms form a NiAs-type arrangement. Further, Cu(2) atoms partially occupy the trigonal-bipyramidal sites formed by Sn7, where the hexagonal unit cell attains an axial ratio ch/ah ≈ \(\sqrt {{\raise0.7ex\hbox{$3$} \!\mathord{\left/ {\vphantom {3 2}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{$2$}}}\) (2c, 2a and 2d Wyckoff sites, for Sn, Cu(1) and Cu(2), respectively7), in agreement with the crystal structures of a larger series of phases8. Assigning an average occupancy of δ to the Cu(2) sites, the η phase attains a composition Cu(1)Cu(2)δSn or, in short, Cu1+δSn, where the composition is quantified in terms of the value of the parameter δ. That parameter will be used throughout this work to designate the composition of the intermetallic. In contrast, the alloy composition will be given in terms of molar fractions via formulas of the type Cu100−xSnx. See Table I for details of the crystal structure of the η and of all the further discussed structure variants of the Cu6Sn5 intermetallic.
(a) Part of the phase diagram Cu−Sn as derived in Ref. 11 and shown here after adaption, representing stable (black) and metastable (red) phase equilibria involving the Cu6Sn5 intermetallic showing up as stable η, η′ and metastable η′′ phases. The dashed grey lines depict the phase boundaries in prominent previous versions of the phase diagram implying a polymorphic transition over a larger composition range4, 5. (b) Updated version according to the present work with filled data points from the present work and open ones from previous works: blue representing averaged compositions of η from diffusion couples due to Ref. 10 with revised δ values due to Eq. (1), black/red solvus compositions from equilibrated alloys11 (Color figure online).

adapted from Ref. 21 (Color figure online).
Hexagonal unit cell of the B81/B82 or NiAs/Ni2In type η-Cu1+δSn augmented by further Sn atoms to illustrate the octahedral coordination of the Cu(1) sites by 6 Sn (orange octahedron) and the trigonal-bipyramidal coordination of the Cu(2) sites (light-green trigonal bipyramid; occupied by δ Cu atoms and 1−δ vacancies). The unit cell is spanned by the basis vectors ah, bh and ch (120° angle between ah and bh) with respect to which the different unit cells relevant for the ordered phases can be formulated (see Table I). The red arrows depict the interstitialcy mechanism formulated for diffusion of Cu by (i) moving a Cu(1) atom to a vacant Cu(2) site, followed by (ii) a Cu(2) atom moving into the place of the Cu(1) site vacant due to the previous step (i). Figure
The η′ phase was found to have a monoclinic superstructure9 due to ordering of δ Cu(2) atoms vs. 1−δ vacancies, with the C-centered superstructure cell having a volume which is ten times larger than the volume of the hexagonal unit cell. The ideal superstructure is possible for δ = 0.2, agreeing with the eponymous formula of the intermetallic: Cu6Sn5 (= 5 Cu1.2Sn). Constancy of lattice parameters of η′ generated by interdiffusion of Cu and Sn in the temperature range, in which the η′ phase is stable, was interpreted in terms of a very narrow equilibrium homogeneity range of η′10. Non-equilibrium η′ with δ deviating from 0.2 likely could, however, be obtained by low-temperature annealing of alloys which were previously equilibrated at higher temperatures11.
Some remaining issues concerning the Cu6Sn5 field/intermetallic in the Cu-Sn system were resolved in a series of recent works10,11,12,13:
-
(i)
Following the structural determination of the ordered η′ phase9, further superstructures different from η′ had been reported: η8 and η6 14 as well as η4+1 15. While the former two superstructures imply an (ideal) composition of Cu5Sn4 (δ = 0.25), the latter implies Cu46Sn37 (δ = 9/37 = 0.243). The η4+1 superstructure was also explicitly referred to as a phase, while it was not attempted to include η4+1 as a second ordered phase in the Cu−Sn phase diagram. A new view of the previously reported superstructures η8 and η4+1 was offered in Refs.11, 13 based on powder x-ray diffraction (PXRD) analysis. According to these works, both superstructures are actually approximants of an incommensurate η′′ phase with continuously varying structure parameters. An ideal δ-dependent structure model was derived, whereby, however, the structure parameters determined from PXRD characteristically deviate from the ideal structure model, supporting the incommensurate character of the crystal structure of the η′′ phase.
-
(ii)
Investigation of PXRD data10,11,12 of η, η′ and η′′ at ambient temperature having experienced different heat treatments prior to quenching to ambient temperature reveals significant variations of the positions of the Bragg reflections, being indicative of varying lattice parameters mainly attributable to varying composition of the corresponding phases. For that purpose, the unit cell volume of low-temperature generated η′12 was taken as reference for the composition δ = 0.2, and, furthermore, the composition dependence of the unit cell volumes as predicted by first-principles calculations was adopted and volume changes observed upon polymorphic ordering were considered. Out of this, the following relation has been proposed to hold between the unit cell volume \({V}_{\mathrm{h}}\) referring to two Sn atoms and the value of δ in the corresponding phases:
$$ V_{{\text{h}}} = V_{{\text{h}}}^{*} \left[ {1 + A\left( {\delta - 0.2} \right)} \right] $$(1)where \(V_{{\text{h}}}^{*}\) is a reference value pertaining to δ = 0.2, and \(A = 0.259\)10. In the case of the ordered η′/η′′ phases, \(V_{\text{h}}^{*} = 78.141~{{\AA^3}}\)10,11 was adopted, whereby \(V_{{\text{h}}}\) is either 1/10 of the unit cell volume of the conventional C-centered monoclinic superstructure cell of η′, and ½ of the monoclinic unit cell of the average structure used to describe the commensurately modulated ordering in the η′ phase10 or the incommensurately modulated ordering in the η′′ phase11, cf. Table I. While in Ref. 10 it had been suggested to use the same \(V_{{\text{h}}}^{*}\) value for the disordered η phase, observation of a volume contraction by 0.13% upon ordering of η′′, it was suggested to use a larger value of \(V_{\text{h}}^{*} = 78.243~{{\AA^3}}\) for η11. That latter \(V_{{\text{h}}}^{*}\) value leads to a decrease of 0.005 in the calculated value of δ as compared to the value recommended for η′/η′′.
Note that all the volume values listed refer to a measurement temperature of the lattice parameters at 296 K. The considerations with respect to the small volume change due to varying order and with respect to the quite significant volume change due to varying composition are, however, equally valid at a given elevated temperature, as long as the coefficient of thermal expansion is sufficiently independent of the state of order and of the composition.
-
(iii)
Phase field boundaries in the binary phase diagram were derived based on measured lattice parameters of η- and η′-containing microstructures, in which these phases had been equilibrated with the Sn-rich melt, with (solid) β-Sn or with (ε-)Cu3SnFootnote 110, 11. Furthermore, information was taken from microstructures in which η and η′ coexist in (local) equilibrium. These data have led to the black phase boundaries depicted in Fig. 1a. The η′′ forms evidently below some temperature-dependent transus temperature < 220°C, as a proposed metastable phase.
The present paper reports exploration of the phase transformation processes leading to the stable or metastable equilibria reported in Refs.11, 13 on the microstructural level. While the results largely confirm and partially refine Fig. 1a from Ref. 11, new insight on the mechanisms of the phase transformations is obtained. This insight can be taken as valuable input for modeling of the transformation processes during the heat treatment of Cu6Sn5 intermetallic, such as that occurring during soldering.
Methods/Experimental
Alloy Preparation and Heat Treatment
Alloys of nominal compositions Cu57Sn43 and Cu52Sn48 were prepared mainly following the route described in Ref. 11. All heat treatment steps were conducted in sealed fused silica tubes evacuated and backfilled with argon at a pressure implying approximately 1 atm at the annealing temperature (relevant for the 1073 K treatment). The initial alloys (about 5 g) were prepared from appropriately weighted Cu and Sn pieces and melted at 1073 K for 24 h and cooled by water quenching of the closed tube. The same tube was then heated to 653 K for 120 h followed by quenching in ice water, including immediately crushing the tube. Although the alloys are not single-phase either at the treatment temperature or after quenching, we refer to the treatment at 653 K as homogenization because the η phase attains a specific, homogeneous composition in equilibrium with Cu3Sn (Cu57Sn43) or with the melt (Cu52Sn48). These homogenized alloys were broken into pieces, which were resealed for further heat treatments in a salt bath (240°C/513 K) or in an oil bath at lower temperatures. For safety reasons, the tubes were quenched without immediately breaking them, leading to only moderate cooling rates.
Powder X-ray Diffraction
For PXRD analysis, alloy pieces were crushed and ground in an agate mortar, and the powder was put through a 50 μm/mesh sieve. Measurements were done on a thin powder layer on a (510)-cut Si single crystal. Data were recorded on a Bruker D8 ADVANCE diffractometer equipped with a Co tube and a quartz-crystal Johansson monochromator in the primary beam providing monochromatic Co-Kα1 radiation. The typical specimen temperature during measurement was 296 K. A LYNXEYE position-sensitive detector was used to collect the diffracted intensity in a range of diffraction angles of 2θ = 18–150°. The data were evaluated to determine the state of order of the Cu6Sn5 intermetallic and to determine the lattice parameters of the corresponding η or η′′ phase for composition determination in terms of the value of δ according to Eq. (1). These evaluations were done using the Rietveld technique applied in TOPAS software22, assuming independent pseudo-Voigt functions for each phase and refining a specimen displacement error. In the case of the η phase, the disordered hexagonal structure model was applied also if broad satellite reflections suggested early-stage η′′ formation due to relatively inefficient quenching. In such cases the quality of fit could be improved considerably by refining parameters describing anisotropic microstrain broadening23,24. In the case of the presence of a well-established η′′ phase exhibiting narrow satellite reflections, a disordered structure having the monoclinic average unit cell was used for fitting, thus ignoring the relatively weak satellite reflections in the course of the refinements. Full fits considering the satellite reflections were presented in earlier works11,13. In the present paper, only a part of the PXRD scans is shown to illustrate the most prominent changes in the profiles.
Lattice parameters extracted from the diffraction peaks of η and η′′ by Rietveld refinement (see above) were evaluated by application of Eq. (1) and the respectively appropriate value of \({V}_{\mathrm{h}}^{*}\). A conservative relative error of 10−4 for all lattice parameters a, b and c (the Rietveld precision of the lattice parameters is much smaller) in the same direction implies a relative error in the unit cell volume of 3 × 10-4, leading to a change in the compositional parameter δ of 1.2 × 10−3. Hence, δ values are reported with three post-decimal positions. Such a change in δ implies a compositional change of 0.02 at.% Sn. It is noted that this change is more closely related to precision than accuracy, because the value of δ relies on the validity of Eq. (1). Application of Eq. (1) implies absence of parameters which affect the lattice parameters beyond the phase’s composition and its state of order, like stresses or ternary alloying elements. In particular, the last influence factor is absent in the present study.
In the case of Rietveld fits with two different η phase structures having different sets of lattice parameters, and with coexisting η and η′′, the phase fractions were also extracted for further evaluation.
Scanning Electron Microscopy (SEM)
For SEM analysis, alloy pieces were cold-embedded in epoxy resin to avoid further impact of heat during embedding. After grinding, the samples were finally polished with diamond paste and colloidal silica (40 nm) as a final step to appropriately prepare the surface for electron backscatter diffraction (EBSD) measurements. Further, the specimens were coated with carbon to guarantee electrical conductivity of the sample and the adjacent epoxy surface. A JEOL JSM-7800F field emission gun-SEM was used, equipped with EDAX energy-dispersive X-ray analysis (EDX) and EBSD systems. An acceleration voltage of 20 kV resulted in a beam current of approximately 7 nA. To obtain microstructural information regarding the local distribution of the individual phases and grain size, backscattered electron (BSE) contrast was mainly used for imaging. For the EBSD measurements, a modest binning of 2 × 2 pixels of the Hikari Super camera was applied. All Kikuchi patterns were stored for post-processing with the OIMA software package employing optimized background and Hough-transformation settings. For EBSD indexing, Kikuchi band positions pertaining to the strongest structure factors were calculated from appropriate structure models. For β-Sn, the usual structure with I41/amd space group symmetry and a = 5.83 Å and c = 3.18 Å was adopted25, whereas in the case of (ε-)Cu3Sn a disordered hexagonally closed packed structure with P63/mmc space group symmetry and a = 2.76 Å and c = 4.32 Å was preferred. The latter choice basically corresponds to a disordered version of the superstructures reported in the literature16,17,18,19,20 and avoids the problems associated with distinction between pseudo-symmetric orientations, which is most likely not possible with the available Kikuchi patterns (see also footnote 1). The η phase was described hexagonal with a = 4.22 Å and c = 5.11 Å (P63/mmc symmetry), whereas the η′′ was tentatively described in terms of the average structure with a = 4.22 Å, b = 7.32 Å, c = 5.11 Å and β = 90.23° (C2/c symmetry), not taking into account the atomic ordering (cf. Table I). Attempts to distinguish η and η′′ and the pseudosymmetric orientations of the latter by means of standard Hough-space-based indexing was not possible. All shown EBSD maps were indexed based on the η phase structure.
Results of Alloy Analysis and Preliminary Discussion
Alloys after Homogenization at 653 K
For use in the present study, two Sn-poor Cu57Sn43 and one Sn-rich Cu52Sn48 alloys were prepared and homogenized at 653 K followed by quenching. As expected from the alloy compositions in view of the phase diagram (Fig. 1) and in agreement with Ref. 11, SEM-based microstructure analysis (Fig. 3) and PXRD analysis both indicate that the alloys in the solid state are dual-phase: Cu3Sn + η in the case of Cu57Sn43 and η + β-Sn in the case of Cu52Sn48.
Scanning electron microscopy images recorded using back-scattered electrons of (a, c) Cu57Sn43 and (b, d) Cu52Sn48 after the homogenization treatment at 653 K for 120 h followed by water quenching. The dual-phase microstructures present at the treatment temperature (a, c) Cu3Sn + η and (b, d) η + melt comply with the phase diagram (see Fig. 1), where the solidified melt, marked by β-Sn in (b), actually consists of η + β-Sn (d). In (a, c) the pores (P in (c)) are parallel to the original Cu3Sn dendrites.
In the case of the Cu57Sn43 alloys, the phase composition is that which is already present at the homogenization temperature. The usually somewhat elongated Cu3Sn particles (alongside with pores, see Fig. 3a, c) embedded into η appear to be arranged in colonies of parallel lines. EBSD analysis reveals that, within a colony, these Cu3Sn particles have the same crystallographic orientation (see Figure S1(a–c) in supplementary material). This can be understood based on the as-solidified microstructure obtained upon quenching, which shows large colonies of Cu3Sn covered by peritectically formed η-phase rims (see Figure S2 in supplementary material). The Cu3Sn particles in the homogenized alloys are obviously remainders of these dendrites in a partially spheroidized shape.
In the case of the more Sn-rich Cu52Sn48 alloy, η coexisted with Sn-rich melt at the homogenization temperature. The presence of this melt leads to more long-range material transport during the annealing. Large, non-faceted η particles developed which are embedded in the Sn-rich melt at the homogenization temperature, see Fig. 3b, d. EBSD analysis (see Figure S1(d–f) in supplementary material) confirmed the single-crystal character of these η particles. The rare η-η grain boundaries form triple junctions with the melt, where the latter assumes a very small angle, indicating a very good wetting of the η phase by the melt. The melt had solidified upon quenching, producing likely Sn-rich η phase particles embedded in the melt (see Fig. 3d). We assume that this η formed upon solidification (see Fig. 1) is more Sn-rich than the main η equilibrated at the annealing temperature with the melt, and that its contribution to the PXRD patterns is small so that its presence can be neglected.
The three homogenized alloys give PXRD data well compatible with the patterns shown in Ref. 11 from similar alloys. The lattice parameters evaluated yield, upon use of Eq. (1), compositions of the η phase of Cu1.237Sn (used for heat treatments at 513 K) and Cu1.235Sn (used heat treatments at 453–483 K) for the two Cu57Sn43 alloys and Cu1.234Sn for the Cu52Sn48 alloy. Due to the different two-phase equilibria, the Cu52Sn48 alloy should indeed contain an η-phase with more Sn than the Cu57Sn43 alloys, although the difference is expected to be small (see Fig. 1a). The slightly different η-phase compositions obtained for the two alloys of Cu57Sn43, however, likely reflect the limitations in accuracy of the lattice parameter determination (in the order of 10−5) but possibly also of the reproducibility of the heat treatment and quenching procedures.
Composition Change by Annealing at 513 K
As already shown in Ref. 11, heat treatment of the alloys quenched from 653 K, at lower temperatures (593 K and 533 K), leads to an increase in the Sn content in the η phase, i.e. a decrease in the value of δ. Lattice parameters determined from correspondingly long-term treated and quenched alloys were a source for δ values which had been used to draw the solvus lines for the Cu3Sn+η/η and η/η+melt, see Fig. 1. The present heat treatments at 513 K for different periods of times were performed to reveal the time-dependent behavior of the alloys and to characterize the transformation on a microstructural level based on alloys having experienced intermediated stages of transformation.
The PXRD data taken from Cu57Sn43 (Cu1.237Sn batch) and Cu52Sn48 heat-treated for different periods of time at 513 K reveal, for both alloys, shifts of the Bragg reflections towards high angles, see Fig. 4. These shifts imply decreasing lattice parameters due to increasing Sn content and decreasing values of δ in view of Eq. (1), as expected for establishment of the new Sn content in the η phase in equilibrium with Cu3Sn (Cu57Sn43) and Sn-rich melt (Cu52Sn48) according to the mentioned temperature-dependent solvus lines. While at intermediate treatment times the reflections in the PXRD data generally appear broadened due to compositional inhomogeneity24,26,27, these reflections become narrow again due to establishment of the new equilibrium.
Part of the PXRD data taken with Co-Kα1 radiation from (a) Cu57Sn43 and (b) Cu52Sn48 alloys, respectively, after homogenization at 653 K for 120 h as well as after subsequent treatment at 513 K for the indicated periods of time. The Sn enrichment of the η phase is reflected by the gradual shift of the reflections 102h and 110h to high angles. Comparison is eased by the vertical blue dashed lines at the peak maxima from the as-homogenized alloys. Reflections of the non-Cu6Sn5 Cu3Sn and β-Sn have been indicated, as well as a broad satellite reflection due to η′′ developed during insufficient quenching (S; 0121 reflection of the incommensurate description from Ref. 11, 13).
Thereby, the following reactions are implied:
in the case of Cu57Sn43, initially already containing Cu3Sn, as well as
in the case of Cu52Sn48, containing an excess of Sn-rich melt. As noted in the Alloys After Homogenization at 653 K Section, in both cases reflections of the respective minority phases Cu3Sn (Cu57Sn43) and β-Sn (Cu52Sn48) were visible in the PXRD data (see e.g. Ref. 11). Estimated changes in the phase fractions of these phases are, however, small and thus cannot be used reliably for estimating the progress of the transformation.
While, however, in the case of the Cu57Sn43 the composition distribution as reflected by the shifting Bragg peaks appears to remain unimodal, in the case of the Cu52Sn48, intermediate states show split peaks. Hence, the PXRD data in the case of Cu52Sn48 were evaluated in terms of two different η phases, giving two independent composition values δH and δL and refined phase fractions. Average composition values \( \overline{\delta } \) were calculated by weighting δH and δL with the respectively refined phase fractions. The different types of δ have been compiled for both alloys in Fig. 5, confirming that the compositions of the η phase asymptotically approach the values due to the solvus lines.
Composition change of the η phase as assessed from the unit cell volumes determined based on PXRD data due to annealing of, respectively homogenized, Cu57Sn43 (black) and Cu52Sn48 (red) alloy at 513 K for different times. Note the changed spread of the time axis at t = 2 h and 250 h. Compositions are given in terms of the value δ calculated by use of Eq. (1). The squares at 0 h feature the values for the alloys quenched from 653 K, and the circles and triangles represent the data for the heat-treated alloys. Due to the split Bragg peaks encountered for heat-treated Cu52Sn48, two values, δH and δL, were determined. The numbers at these data points are the refined molar fractions (referring to the number of atoms), which were used to calculate the weighted average \({\bar{\delta}}\). The full lines are the fits according to Eq. (4) and the parameters according to Table II, with the arrows highlighting the systematic deviations of the measured data to the fit in the case of the Cu52Sn48 alloy. The dashed lines for the δH and δL have been indicated to guide the eyes (Color figure online).
The peak splitting observed for intermediate treatment times of the Cu52Sn48 alloys might be related to the particle size distribution leading to rapid Sn enrichment, in particular of small particles. Interpretation of the data is, however, obstructed by the limited penetration depth of the x-rays into the η phase, which is much smaller than the size of the powder particles, even after powdering and sieving. Indeed, while the attenuation coefficient due to x-ray absorption amounts to about 0.2 μm-1 ((5 μm)−1), the powdered and sieved particles may still have a diameter in the order of a few 10 μm. The PXRD data of both Cu57Sn43 and Cu52Sn48 alloys, heat treated for the shortest times of 1 h, show a broadening of the (102)h reflection of η to low angles, which is not expected in view of the general trend of a high-angle shift of all η reflections due to the depletion of the η by Cu. Emergence of broad satellite reflections (see Fig. 4) due to the formation of η′′, however, indicated that quenching after taking the alloys out of the salt bath (see section Alloy Preparation and Heat Treatment) was not sufficiently rapid to suppress ordering completely. This η′′ formation was associated with a monoclinic distortion of the pseudo-orthorhombic average structure evaluated in Ref. 13 (see Table I). Upon referring the reflections to a hexagonal unit cell, one expects a microstrain distribution due to the emerging lattice distortion associated with η′′ ordering with a large \( \left\langle {\varepsilon _{{13}}^{2} } \right\rangle \) component of the covariance tensor describing this distribution. This leads, in view of Ref. 28, to a large positive value of S202 (or equivalently Z1313) for the typical descriptions of microstrain broadening in the course of Rietveld refinement22. This is indeed observed for the treatment times of 1 h for both alloys. For the longer treatment times this special type of line broadening seems absent, which is compatible with the absence of η′′ satellite reflections in spite of the same slow quenching procedure. Encountering the microstrain broadening for only the shortest treatment times is compatible with the composition dependence of the transus temperature η\( \rightleftarrows \)η′′ shown in Fig. 1. In Ref. 11, the decrease of the transus with increasing Sn content had only tentatively been suggested. This composition dependence implies that for higher Sn content, the ordering starts at lower temperature during cooling such that the applied quenching rate is sufficient to avoid emergence of appreciable ordering. Moreover, the η phase enters the region of η′ ordering with increasing Sn content, which shows a much lesser degree of unit cell distortion than η′′13, hence leading to a lesser degree of line broadening even if ordering occurs. Note that the ordering kinetics towards η′′ (referred to as η4+1) and η′ have been studied29,30,31 without revealing the actual course of the effects as a function of composition of the intermetallic such that the particular composition dependence of the ordering/transus temperature was not revealed. In any case, the data29 reveal a very rapid formation of η′′ ordering, which is most rapid at about 450 K, being likely sufficient to allow order formation for the Sn-poor η phase upon the applied slow quenching.
Microstructure analysis of alloys treated for intermediated times by SEM (see Fig. 6) reveals details on the mechanism of the compositional change of the η phase.
Scanning electron microscopy analysis of (a–c) Cu57Sn43 (one scale bar for a and b, the area shown in (c) corresponds to the rectangle in (b)) and (d) Cu52Sn48 alloys, respectively, previously homogenized at 653 K for 120 h and further subjected to heat treatment at 513 K for 1 h (Cu57Sn43) and 30 h (Cu52Sn48). (a) BSE image showing Cu3Sn formed at grain boundaries during the treatment at 513 K. (b, c) EBSD map of other region with inverse pole figure coloring for regions indexed as Cu3Sn and image quality highlighting the newly nucleated Cu3Sn at the grain boundaries and the growth of already existing Cu3Sn particles along the grain boundaries. (d) BSE image showing spatially varying backscatter intensity within the η phase: light for Cu-poor/Sn-rich regions, dark for (original) Cu-rich/Sn-poor regions. EDX-based Sn contents measured along the dashed line (white data points and red smoothed curve) are superposed (Color figure online).
For the Sn-poor Cu57Sn43 alloy having experienced a short heat treatment time, Cu3Sn appears in morphologies which had not been observed in the corresponding alloy equilibrated at 653 K and quenched (see Fig. 3a, c and Figure S1a–c). This new Cu3Sn (i) covers the grain boundaries of the η phase (see Fig. 6a–c) and (ii) appears as elongations of old Cu3Sn particles along grain boundaries (see arrows in Fig. 6b). Obviously, the grain boundaries are preferred nucleation sites for the Cu3Sn and regions of accelerated mass transport, as also discussed recently32.
For the Sn-rich Cu52Sn48 having experienced an intermediate heat treatment time (see Fig. 6d), the decrease of the Sn content revealed by the PXRD data is reflected by a tiny increase of the backscatter electron intensity at the rims of the η phase due to the expected increase of the Sn content (i.e. in contact with the Sn-rich melt) and at grain boundaries of the η phase. An EDX scan confirms the heterogeneity implied by the backscatter electron contrast (see Figs. 6e vs. d). Note, however, not all regions close to the grain boundaries or directly adjacent to the Sn-rich melt reveal the same increase of the backscattered intensity, which might be related to anisotropy of the diffusion or due to the unknown three-dimensional arrangement of the microstructure visible in the two-dimensional section.
In the Cu52Sn48 alloy, no Cu3Sn according to Eq. (2) develops, either inside the large η grains or at the η-grain boundaries. Such Cu3Sn formation might occur as an intermediate way of approaching local equilibrium if Cu3Sn nucleation would be successful and if the excess-Sn from the melt has not made its way to the corresponding regions. Non-detection of such intermediate Cu3Sn in this alloy indicates (i) the high permeability of the grain boundaries for diffusion concluded from diffusion/phase growth studies32 and as also implied by the wetting behavior mentioned in the section Alloys After Homogenization at 653 K, and (ii) the difficulty of Cu3Sn nucleation at places other than grain boundaries.
Evaluation of Diffusion Coefficient
Interdiffusion coefficients in η can be estimated from the kinetic data shown in Fig. 5, if one assumes that the rate of the composition change in η is determined by (volume) interdiffusion in that phase, exchanging Cu/Sn with the Cu3Sn or Sn at the grain/phase boundaries. As a model to describe the diffusion kinetics, one can take the well-established case of diffusion into (or out of) spheres of radius R (to be associated with the η-grain radius in the respective alloys) with a composition-independent interdiffusion coefficient D. In that case it holds33:
Thereby, \(M\left( \infty \right)\) is the total amount of material to be exchanged between spheres and their environment (attained at time t = ∞), whereas M(t) is the corresponding value attained after the time t. The ratio \(M\left(t\right)/M\left(\right)\) varies between 0 and 1 in the course of the process and is typically related linearly with the relative change of the average concentration (amount per volume) of a component in the spheres. Due to the very small volume changes upon composition change, one can consider, instead of the change of concentration, either the change of the molar fractions or, as in Eq. (4), the change of the parameter δ. Thereby, \(\delta \left(0\right)\) is the value of the alloy after quenching from 653 K, \(\delta \left(0\right)\) is the value at t = ∞, which evidently differs for the two alloys. \(\delta \left(t\right)\) is the average value of δ after annealing for the time t. This value \(\delta \left(t\right)\) will be associated with the values determined by PXRD and as given in Fig. 5, whereby in the case of Cu57Sn43 the value of \( \overline{\delta } \) will be adopted.
For the datasets \(\delta \left(t\right)\) from both alloys, least squares fitting of the ratio D/R2 was done using the data points at t > 0 and adopting n = 1–16 in Eq. (4).Footnote 2\(\delta \left(0\right)\) and \(\delta \left(\infty\right)\) are taken from the as-homogenized alloy and from the alloy having experienced the longest treatment time. The fitted values of D/R2 are contained in Table II.
In the case of the Cu57Sn43 alloy, the fit of Eq. (4) to the \({\delta }\left(t\right)\) data is quite reasonable (see black unbroken line, Fig. 5). Hence, the fitted value of D/R2 is used to calculate an interdiffusion coefficient. For that, a value of the radius R of the η grains was determined using the line intersection method using SEM images like those in Fig. 3a, c. Counting the number of intercepts (η grain boundaries and phase boundaries with Cu3Sn) on a grid superimposed with the BSD image of the η/Cu3Sn microstructure gave a grain radius of (21 ± 4) µm. Based on this, an interdiffusion coefficient of (3 ± 1) × 10-16 m2s−1 was determined, with an error dominated by the spread of grain sizes.
In the case of the Cu52Sn48 alloy, the corresponding fit of Eq. (4) to the \(\stackrel{-}{\delta }\left(t\right)\) data is considerably poorer (see red unbroken line in Fig. 5 with deviations highlighted by arrows). This poorer fit can be attributed to the inadequacy of Eq. (4) for a (wide) distribution of particle/grain (sphere) sizes. That size distribution is evidently much broader in the case of the Cu52Sn48 alloy than in the case of the Cu57Sn43 alloy (see Fig. 3), leading to a relatively rapid saturation of the small particles and slow saturation of the large particles, readily explaining the systematic deviation of the fitted curve from the data points evident in Fig. 5. Due to these deviations in the case of the Cu52Sn48 alloy, we refrained from interpreting the fitted value of D/R2 in terms of an interdiffusion coefficient based on an estimated average grain size. But, it appears understandable in view of the fraction of the very large grains visible in Fig. 3b, d that it takes much longer to attain equilibrium in the case of the Cu52Sn48 alloy than in the case of the Cu57Sn43 alloy.
η″ Formation and Composition Change by Annealing at 453–483 K
PXRD patterns of Cu57Sn43 alloy pieces homogenized at 653 K (Cu1.235Sn batch) and afterwards annealed at 453 K, 463 K, 473 K and 483 K for 24 h, respectively, show clear evidence for the formation of the η′′ phase, as evidenced by satellite reflections and also by the splitting of, in particular, the 102h reflection, see Fig. 7a. Note that the satellite reflections thus formed are much narrower than those formed due to insufficient quenching from 513 K (see section Composition Change by Annealing at 513 K and Fig. 4), indicating that the order evidenced by the PXRD data in Fig. 7a has developed during the heat treatment.
Part of the PXRD data taken with Co-Kα1 radiation from Cu57Sn43 alloy (containing Cu3Sn as non-Cu6Sn5 phase) after homogenization at 653 K for 120 h as well as after subsequent treatment (a) at 453–483 K for 24 h and (b) at 473 K for the indicated period of time. In comparison with the as-homogenized state, the treatments induce η′′ order indicated by satellite reflections (S) and the splitting (into three) of the 102h reflection (highlighted by the red circles). Annealing at increased temperature (a) and for prolonged time (b) leads to progressing Sn enrichment of the Cu6Sn5 intermetallic accompanied by weakening η′′ order (weaker satellite reflections S) and decreasing reflection splitting including formation of Sn-rich(er) η phase evidence by changed shape of the 102h reflection (blue circles). Comparison is eased by the vertical blue dashed lines at the peak maxima from the as-homogenized alloy (Color figure online).
In the case of annealing at 453 K, the 102h reflection splits into three peaks of approximately the same intensity (indices \(11\overline{2}\) av, 022av, \({112} _{{{\text{av}}}}\) with respect to the unit cell of the average cell, see Table I), in agreement with patterns of alloys annealed at even lower temperatures.11,13Footnote 3 In the case of the alloys which experienced annealing at higher temperatures for the same period of time, the high-angle side of the 102h reflection (group) gets stronger. Qualitatively similar results as observed with increasing treatment temperature at 24 h are encountered upon annealing at constant 473 K for an increasing period of time (see Fig. 7b). Evidently, the diffraction patterns approach the patterns of the disordered η phase. This can be understood as follows: the value of the transus temperature (order-disorder η\(\rightleftarrows\)η′′ transformation temperature at constant composition) for the composition of the quenched η (Cu1.235Sn) is higher than the highest annealing temperature of 483 K applied for this series of experiments. As implied by the phase diagram shown in Fig. 1, the composition of the intermetallic alloy (for the employed Cu57Sn43 batch) is located in the two-phase region Cu3Sn + η and tends to decompose, as was also observed for annealing at 513 K (see section Composition Change by Annealing at 513 K). Hence, likewise Cu3Sn will develop while increasing the Sn content of the remaining Cu6Sn5 intermetallic. Due to the composition dependent η\(\rightleftarrows\)η′′ transus, those parts of the Cu6Sn5 intermetallic which have attained a lower Sn content get disordered at the annealing temperature, while the more Sn-poor intermetallic may still have an η′′-like order. The process becomes slower with decreasing temperature and proceeds with increasing treatment time. We refrain from reporting results from quantitative fitting based on these complex patterns due to the severe reflection overlap and the correlations of the refined parameters involved. In the context of this evaluation, it appears that the volume difference upon ordering at 453 K-483 K is somewhat smaller than for ordering at 438 K11, likely due to the temperature dependence of the order parameter, such as that reported for structurally related incommensurate LT′-Ni1.35-41Sn21,34,35.
In any case, the very rapid η′′ order formation at 473 K is highly compatible with the findings from Ref. 29. The treatment times in that work were, however, too short to reveal the slow increase of the Sn content in the Cu6Sn5 intermetallic upon long-term treatment as they have been observed here and which have led to disordering by crossing the transus temperature during annealing at 473 K.
SEM imaging of the alloy heat treated for 24 h at 453 K using backscattered electrons reveals a substructure within the original η-phase grains (see Fig. 8), which is not present for the Cu6Sn5 intermetallic quenched from 513 K and above. As indicated in the section Domain Formation in η, this is an effect of the ordered domains.
Scanning electron microscopy images taken with backscattered electron intensity of Cu57Sn43 alloy previously homogenized at 653 K for 120 h and further heat treated at 453 K for 24 h to generate η′′ order. Contrast was strongly enhanced to reveal the orientation contrast due to the twin domains, such that the Cu3Sn appears black. (a) overview, (b) detail, from another place of the specimen.
Overall Discussion
Diffusivity
It has been concluded from detailed studies on anisotropic volume tracer diffusion of Ni and Sn in structurally analogous Ni3Sn2 (Ni1+δSn) intermetallic36,37 that (i) Ni is much more mobile than Sn with a higher activation energy of the latter, and (ii) that Ni moves by an interstitialcy mechanism involving both Ni(1) and Ni(2), which can drive exchange of Ni(2) with vacancies on trigonal-bipyramidal sites. That knowledge about Ni mobility (point (ii)) was employed to interpret the kinetics of order formation and domain coarsening in the Ni1+δSn intermetallic33,34.
In contrast, little direct information is available on the actual atomic processes associated with composition change and order formation in the Cu6Sn5 intermetallic. For order formation, it is sufficient that the Cu(2) atoms are able to exchange sites with vacancies on the trigonal-bipyramidal sites, whereas for interdiffusion-driven long-range composition change, both Cu and Sn can contribute. It is likely against the background of the knowledge on the structurally analogous Ni3Sn2 (Ni1+δSn) intermetallic that Cu transport in Cu1+δSn occurs by an analogous interstitialcy mechanism (indicated by the red arrows in Fig. 2) and that Cu is more mobile in the volume than Sn.
Kinetic information on the Cu6Sn5 intermetallic exists from the study of order formation and from layer growth kinetics. The kinetics of order formation for η′ and η4+1 (here identified as η′′) out of η have been analyzed quite systematically, but quantitative characteristics like activation energies have not been reported29. A large body of data on diffusion-related quantities exists from measuring growth kinetics of Cu6Sn5 intermetallic, e.g.32,38,39,40,41,42. Most data evaluated in terms of actual diffusion coefficients exist for temperatures below the melting point of Sn, because just above the melting point a pronounced scallop-like growth of the Cu6Sn5 intermetallic (as η phase) obstructs determination of meaningful diffusion coefficients by conventional analysis of layer growth43,44,45,46. Due to the small and uncertain homogeneity range of the Cu6Sn5 intermetallic (see Fig. 1), frequently only integrated interdiffusion coefficients have been determined, e.g.38(based on parabolic growth constants measured in the range 423–473 K). The relatively small value of the activation energy 81 kJ mol−1 was attributed to a significant contribution of grain boundary diffusion to the growth of the Cu6Sn5 intermetallic. In the same work38, observation of the positions of Kirkendall markers revealed a significant contribution from both Cu and Sn diffusion to the overall interdiffusion, apparently contradicting the much larger tracer diffusion coefficients of Ni as compared to Sn in analogous Ni3Sn2 (Ni1+δSn) intermetallic (Refs. 36, 37, see above). This contradiction can, however, be resolved by assuming that a considerable contribution from Sn grain-boundary diffusion is responsible for the contribution of Sn to the observed overall interdiffusion during layer growth under “solid-Sn” conditions.
A somewhat lower activation energy of 65 kJ mol−1 was reported earlier for real interdiffusion coefficients assessed under solid-Sn conditions42 (as determined for a temperature range of 463–493 K). The temperature-dependent evolution is indicated in Fig. 9 alongside the value determined for 513 K in the section Evaluation of Diffusion Coefficient (see Table II). Evidently, that value is smaller by a factor of 10 than the value extrapolated from low temperatures42. Thereby, it has to be noted that the data in Ref. 42 had been evaluated taking into account a homogeneity range of a width as indicted in Fig. 1a in grey (Ref. 6 has been cited as a source in Ref. 42), which is larger than the homogeneity range in recent works10,11 (black boundaries in Fig. 1a). If such a smaller homogeneity range had been used to determine the interdiffusion coefficients from the parabolic growth constant data42, the interdiffusion coefficient from42 would have been even larger. The lower value of the interdiffusion coefficient at 513 K determined in the section Evaluation of Diffusion Coefficient as compared to the extrapolated interdiffusion coefficient from42 can, however, be explained by the absence of the grain-boundary diffusion contributions but sole presence of volume contribution in the former case.
Interdiffusion coefficient determined in the section Evaluation of Diffusion Coefficient of this work (red data point; compare Table II) in comparison with data from the literature determined from layer-growth kinetics (Onishi and Fujibuchi42 and Gupta and Rathor40) determined in the ranges of temperature, where the lines are unbroken, whereas broken lines indicate extrapolation to low and high temperature. The interdiffusion coefficients determined at low temperatures are likely affected by grain-boundary diffusion, while those values determined at high temperatures likely represent volume diffusion. The latter data agree with the value (data point) determined in the present work (Color figure online).
This view appears to be confirmed by comparison of the presently determined interdiffusion coefficient with values determined from layer growth kinetics data at 640 K-673 K41, i.e. just below the peritectic decomposition temperature of the η phase. These interdiffusion coefficients have also been included in Fig. 9 in the form of the assessed temperature-dependent relation. The determined activation energy of 122 kJ mol−1 is larger than those determined for the lower temperatures, which might imply that the corresponding interdiffusion coefficient derived from high-temperature layer-growth kinetics41 is, in fact, due to volume diffusion in η. The assessed temperature-dependent interdiffusion coefficient extrapolated to 513 K gives an excellent agreement with the interdiffusion coefficient assessed in the section Evaluation of Diffusion Coefficient (in contrast to the data from42). This makes it likely that also the value determined in the section Evaluation of Diffusion Coefficient is indeed due to volume diffusion, in view of Refs. 36, 37, likely dominated by diffusion of Cu.
Domain Formation in η′′
Symmetry-reducing phase transitions can lead to the formation of antiphase (translational) and twin (orientation) domains in a transformed volume47,48,49. Occurrence of orientation domains in symmetry-reducing phase transformations is governed by the ratio \(\left| {\boldsymbol{\mathcal{G}}^{\eta } } \right|/\left| {\boldsymbol{\mathcal{G}}^{{\eta ^{{''}} }} } \right| \) of the orders of crystal classes (crystallographic point group, \({\boldsymbol{\mathcal{G}}}\)) of the parent \({\boldsymbol{\mathcal{G}}^{\eta}}\) and the child \( {\boldsymbol{\mathcal{G}}^{{\eta ^{{''}} }} }\) phases. Footnote 4\({\boldsymbol{\mathcal{G}}^{\eta}}\) of the parent disordered η phase is 6/mmm with its order \(\left| {\boldsymbol{\mathcal{G}}^{\eta}} \right| = 24\), while \( {\boldsymbol{\mathcal{G}}^{{\eta ^{{''}} }} }\) is 2/m with \( \left| {\boldsymbol{\mathcal{G}}^{{\eta ^{{''}} }} } \right| = 4\). Hence, \(\left| {\boldsymbol{\mathcal{G}}^{\eta }} \right|/\left| {\boldsymbol{\mathcal{G}}^{{\eta ^{{''}} }} } \right| = 6\), whereby both crystal classes contain a center of inversion such that inversion or polar domains, which are difficult to distinguish by EBSD analysis, do not arise. The images in Fig. 8 do not permit reliable counting of the number of different domain (states) per parent-grain orientation, so that the number of six domain states cannot be confirmed at present. The authors are, however, confident that the contrast variations within Fig. 8 are due to corresponding orientation domains.
EBSD measurements evaluated by standard Hough-space-based indexing, using band positions based on the lattice parameters of the monoclinic average structure, on the first view allowed distinguishing the different pseudosymmetric solutions. These differ by rotations of 60° around the hexagonal c axis of the η phase, i.e. they are interconverted by the six-fold rotation axis which has been lost during symmetry reduction. Direct “visual” inspection of the calculated and observed band positions, however, implied the unreliability of this discrimination of different domain orientations, obviously due to biasing by the settings of the band detection and the applied threshold of allowed angular accuracy. Due to the smaller deviatoric strains12, we suspect that domain orientation discrimination in the more frequently encountered η′ phase is even more difficult than in the case of η′′.
Consequences for Heat Treatment Behavior of Cu6Sn5 Intermetallic
The present experimental observations can only be interpreted in view of the fact that each of the three phases (η, η′, η′′) constituting the Cu6Sn5 field according to Fig. 1 can exist either stably or metastably in a finite compositional range, expressed in the present work in terms of the value of δ in a chemical formula of the type Cu1+δSn. Quite naturally, a maximum compositional range can be encountered for the disordered η. While the maximum δ is found as nearly 0.24 (including the alloys equilibrated at 653 K in the present work), its minimum encountered value seems to be about 0.17 in the η phase in the lower limit of its range of stability according to Ref. 10. The maximum and minimum values of the lattice parameters ah and ch (as measured at ambient temperature) are 4.223 Å and 5.113 Å for the present Cu1.237Sn and 4.196 Å and 5.086 Å12, leading to Cu1.166Sn according to Eq. (1) with the presently adopted value of \(V_{{\text{h}}}^{*} = 78.243~{\AA^3}\) (explaining the different composition listed in Ref. 12). These sets of lattice parameters differ by 0.6% (ah) and 0.5% (ch), implying a volume change (decrease) by 1.8% going from the minimum to the maximum Sn content (again, all data refer to ambient temperature or ideally 296 K). This volume change is of the same magnitude as the proposed 2% volume change of “Cu6Sn5” upon ordering, which had been deduced in Ref. 50 and referred to in a series of later works. However, in the opinion of the present authors, that 2% is physically unrelated to the value of 1.8% volume change due to composition change. Already in Ref. 10 it was argued that this very 2% volume change is likely a result of inappropriate comparison of inaccurate lattice parameter data reported in the literature. As mentioned in the introduction, direct analysis of the volume change upon formation of η′′ order at constant composition (and as assessed at ambient temperature) upon annealing 438 K for 72.5 h implied a contraction by about 0.13%11, where in the section η′′ Formation and Composition Change by Annealing at 453–483 K it has been noted that the magnitude of this contraction could be even smaller upon ordering at > 438 K.
Note, however, that it has been shown in the section η′′ Formation and Composition Change by Annealing at 453–483 K that such low-temperature annealing bringing about the η′′ ordering in Sn-poor η can lead to an increase of the Sn content of the Cu6Sn5 intermetallic due to formation of Cu3Sn, i.e. to a decrease of the corresponding value of δ. Occurrence of such decomposition accompanying ordering could invalidate a measured degree of volume contraction due to ordering, because it then would contain a contribution due to composition change. In view of the effects observed in the section η′′ Formation and Composition Change by Annealing at 453–483 K, it can, however, be assumed that composition change due to Cu3Sn formation is sufficiently slow at 438 K for the applied treatment time.
It should further be noted that recently reported high-temperature PXRD data revealed a volume decrease of 0.7 at.% upon disordering η′ toward η upon heating51. Assuming peritectoid equilibrium decomposition of η′ to Cu3Sn + η (the latter richer in Sn than the assumedly stoichiometric η′) as indicated in Fig. 1, a smaller unit cell volume for η is expected. Indeed, the η phase in diffusion couples quenched from the temperature range exhibiting an η′+η two-phase region has a unit cell volume Vh, being about 1 % smaller than that of the coexisting η′.
All this evidence indicates that while the volume change upon ordering of the Cu6Sn5 intermetallic at constant composition is very small (0.13% contraction or smaller), much larger volume changes may occur due to composition changes of the Cu6Sn5 intermetallic resulting from the reactions that can accompany the change in the state order, or which can even occur without changing the state of order. The course of these reactions will depend on the composition of the intermetallic and the heat-treatment program. Hence, the situation is more complicated than if order–disorder transitions would occur at constant composition, as implicitly or explicitly assumed in many works in the past.
As a consequence, the potentially detrimental effects on the mechanical properties of the intermetallic induced by the volume changes, e.g. in a solder joint, will depend on the complicated course of the transformations accompanying the order change and will depend on heating/cooling conditions and the presence of excess Sn or excess Cu. Here a few issues for future works can be identified:
-
(i)
The order-disorder transformation at constant composition from η to the ordered η′ or η′′ and the associated local (admittedly small) transformation strains are compensated by the formation of the observed domain microstructure. But remaining residual stress fields may be present.
-
(ii)
Cooling of the Sn-poor η phase to low temperatures will, in the absence of a Sn source, produce Cu3Sn at grain boundaries upon passing the solvus curve for Cu3Sn+η/η′, which may obstruct grain boundary cohesion in polycrystalline η, in particular relevant if all Sn is used up.
-
(iii)
Local composition changes induced by bulk diffusion in η due to the processes described in (ii) or, alternatively, by the uptake of excess Sn may introduce metrical misfit and stress as a possible source of failure.
In the light of the present results, a slightly modified version of the phase diagram in the Cu6Sn5 region is offered in Fig. 1b. Thereby, the data pertaining to the η from10 (blue points in Fig. 1b) have been modified by considering \(V_{{\text{h}}}^{*} = 78.243~{\AA^3}\) for η, which was introduced in Ref. 11. Without that, the phase diagram offered therein was redrawn in this region. Hence, the eutectoid η→ η′+β-Sn is shifted to higher Sn content. It must, however, be noted that the contents determined10 were averages from layers in diffusion couples. Due to the surface sensitivity of x-ray diffraction, the composition values were associated with the η/η+β-Sn solvus.
Moreover, the final Sn contents pertaining to the Cu3Sn+η/η and η/η+melt solvus at 513 K have been included in Fig. 1b. Notably, the filled red data point due to the η/η+melt solvus of the present work is at significantly lower/higher Sn content than the value determined for 533 K from Ref. 11 (unfilled data point). In that work, however, the heat treatment of 139 h might not have been sufficient to attain equilibrium at 533 K. Hence, preference has been given to the data point at 513 K.
It is finally to be emphasized that the phenomena observed here and the phase diagram are valid for the binary Cu−Sn system. The presence of other elements may, of course, more or less shift the phase boundaries, and will definitely make Eq. (1) unreliable. The present knowledge developed here, however, can be used as starting point for investigation of more chemically complex systems.
Summary and Conclusions
Phase transformations involving the Cu6Sn5 intermetallic in the Cu−Sn system with its phases η, η′ and η′′, occurring due to the temperature-dependent changing phase equilibria were studied by PXRD and SEM. That was done on Cu57Sn43 and Cu52Sn48 alloys, which were previously equilibrated at 653 K. At this temperature, the phase fields are located in the two-phase region ε-Cu3Sn + disordered η in the former and disordered η + Sn-rich melt in the latter. In both cases, after quenching, the η was relatively Sn poor, but was contained in quite different microstructures. By subsequent annealing at lower temperatures for various times, the following processes were induced:
-
(i)
Annealing at 513 K produced a gradual increase in the Sn content in the η phase due to the shapes of the solvus curves. In the case of the Cu57Sn43 alloy new Cu3Sn developed at grain boundaries of η and at already existing Cu3Sn, while it was never encountered in the η-grain interior. In the case of the Cu52Sn48 alloy, gradual uptake of Sn from the Sn-rich melt embedding the η was encountered.
-
(ii)
While Cu57Sn43 alloy heat treated at 483 K and below becomes ordered to the η′′ phase (at the quenched η phase composition of Cu1.235Sn), the unmixing increases the Sn content of the Cu6Sn5 intermetallic, which is compensated by Cu3Sn formation like at 513 K. The rate is of this composition change is, however, considerably slower due to the lower treatment temperature. This finding confirms the previously proposed metastability of the η′′ phase with respect to precipitation of Cu3Sn and that the transus temperature decreases with increasing Sn content.
-
(iii)
Orientational domains of the monoclinic η′′ phase, which developed from the hexagonal η phase upon ordering, have been made visible by SEM in backscattered electron contrast.
The different types of subprocesses (precipitation, volume diffusion, grain-boundary diffusion) involved, including in particular compositional change of the intermetallic, have to be explicitly considered in order to understand or model the processes occurring upon heat treatment of real solder joints. Moreover, the compositional and phase transition information is the basis for understanding the more complex phenomena involving the Cu6Sn5 intermetallic in the presence of further alloying elements.
Notes
By this, the poor convergence of the sum in Eq. (4) in the case of t = 0 is considered. The data at t = 0 are implicitly considered by adopting the values for \(\delta \left(0\right)\).
It should be noted that visibility of this splitting strongly depends on the instrumental resolution function of the diffraction experiment (which is good in the present case, due to the use of CoKα1 radiation) as well as from presence of additional sources of line broadening, like compositional inhomogeneity.
This holds at least as long as the symmetry elements of the child phase, which have been retained from the parent phase (forming the intersection group48) coincide in space. This can usually be assumed to be valid in the case of small strains accompanying the transformation. The largest (deviatoric) component of the strain tensor is only 0.00213, while the distortion of the also monoclinic 12 is even smaller.
References
D.K. Mu, S.D. McDonald, J. Read, H. Huang, and K. Nogita, Curr. Op. Sol. Stat. Mater. Sci. 20, 55 (2016). https://doi.org/10.1016/j.cossms.2015.08.001.
S. Wang, Y. Yao, and X. Long, Appl. Sci. 9, 227 (2019). https://doi.org/10.3390/app9020227.
K. Suganuma, Curr. Op. Sol. Stat. Mater. Sci. 5, 55 (2001). https://doi.org/10.1016/S1359-0286(00)00036-X.
M. Hansen, and K. Anderko, Constitution of Binary Alloys, 2nd ed., (New York: McGraw-Hill, 1958).
N. Saunders, and A.P. Miodownik, Bull. Alloy Phase Diagr. 11, 278 (1990). https://doi.org/10.1007/BF03029299.
T.B. Massalski, and H. Okamoto eds., Binary Alloy Phase Diagrams. (Materials Park, Ohio: ASM International, 1990).
B. Peplinski, G. Schulz, D. Schultze, E. Schierhorn, Mater. Sci. Forum 228-231, 577 (1996). https://doi.org/10.4028/www.scientific.net/MSF.228-231.577
S. Lidin, and A.-K. Larsson, J. Sol. State Chem. 118, 313 (1995). https://doi.org/10.1006/jssc.1995.1350.
A.-K. Larsson, L. Stenberg, and S. Lidin, Acta Crystallogr. B 50, 636 (1994). https://doi.org/10.1107/S0108768194004052.
C. Wieser, W. Hügel, A. Walnsch, and A. Leineweber, J. Electr. Mater. 49, 245 (2020). https://doi.org/10.1007/s11664-019-07643-3.
A. Leineweber, C. Wieser, and W. Hügel, Scr. Mater. 183, 66–70 (2020). https://doi.org/10.1016/j.scriptamat.2020.03.020.
C. Wieser, A. Walnsch, W. Hügel, and A. Leineweber, J. Alloys Compd. 794, 491 (2019). https://doi.org/10.1016/j.jallcom.2019.04.265.
A. Leineweber, C. Wieser, and W. Hügel, Z. Kristallogr. 235, 445 (2020). https://doi.org/10.1515/zkri-2020-0055.
A.-K. Larsson, L. Stenberg, and S. Lidin, Z. Kristallogr. 210, 832 (1995). https://doi.org/10.1524/zkri.1995.210.11.832.
Y.Q. Wu, J.C. Barry, T. Yamamoto, Q.F. Gu, S.D. McDonald, S. Matsumura, H. Huang, and K. Nogita, Acta Mater. 60, 6581 (2012). https://doi.org/10.1016/j.actamat.2012.08.024.
K. Schubert, and W. Burkhardt, Z. Metallkde. 50, 442–452 (1959).
X. Sang, K. Du, and H. Ye, J. Alloys Compd. 469, 129 (2009). https://doi.org/10.1016/j.jallcom.2008.01.107.
H. Knödler, Acta Crystallogr. 10, 86 (1957). https://doi.org/10.1107/S0365110X57000225.
Y. Watanabe, Y. Fujinaga, and H. Iwasaki, Acta Crystallogr. B 39, 306 (1983). https://doi.org/10.1107/S0108768183002451.
C.J. Müller, and S. Lidin, Acta Crystallogr. B 70, 879 (2014). https://doi.org/10.1107/S205252061401806X.
A. Leineweber, J. Sol. State Chem. 177, 1197 (2004). https://doi.org/10.1016/j.jssc.2003.10.028.
R.E. Dinnebier, A. Leineweber, and J.S.O. Evans, Rietveld Refinement (Berlin, Boston: De Gruyter, 2018).
P.W. Stephens, J. Appl. Crystallogr. 32, 281 (1999). https://doi.org/10.1107/S0021889898006001.
A. Leineweber, Z. Kristallogr. 226, 905 (2011). https://doi.org/10.1524/zkri.2011.1413.
M. Wołcyrz, R. Kubiak, and S. Maciejewski, Phys. Stat. Sol. (b) 107, 245 (1981). https://doi.org/10.1002/pssb.2221070125.
A. Leineweber, and E.J. Mittemeijer, J. Appl. Crystallogr. 37, 123 (2004). https://doi.org/10.1107/S0021889803026906.
T.G. Berger, A. Leineweber, E.J. Mittemeijer, and M. Knapp, Z. Kristallogr. Suppl. 23, 443 (2006).
A. Leineweber, Powd. Diff. 32, S35 (2017). https://doi.org/10.1017/S0885715617000665.
G. Zeng, S.D. McDonald, J.J. Read, Q. Gu, and K. Nogita, Acta Mater. 69, 135 (2014). https://doi.org/10.1016/j.actamat.2014.01.027.
K. Nogita, C.M. Gourlay, S.D. McDonald, Y.Q. Wu, J. Read, and Q.F. Gu, Scr. Mater. 65, 922 (2011). https://doi.org/10.1016/j.scriptamat.2011.07.058.
F. Somidin, H. Maeno, M.A.A. Mohd Salleh, X.Q. Tran, S.D. McDonald, S. Matsumura, and K. Nogita, Mater. Charact. 138, 113 (2018). https://doi.org/10.1016/j.matchar.2018.02.006.
A. Kodentsov, J. Wojewoda-Budka, L. Litynska-Dobrzynska, P. Zieba, and A. Wierzbicka-Miernik, J. Alloys Compd. 858, 157677 (2021). https://doi.org/10.1016/j.jallcom.2020.157677.
H. Mehrer, Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes (Berlin: Springer, 2007).
A. Leineweber, Int. J. Mater. Res. 102, 861 (2011). https://doi.org/10.3139/146.110540.
A. Leineweber, and E.J. Mittemeijer, Z. Kristallogr. 222, 150 (2007). https://doi.org/10.1524/zkri.2007.222.3-4.150.
H. Schmidt, G. Frohberg, W. Miekeley, and H. Wever, Phys. Stat. Sol. (b) 171, 29 (1992). https://doi.org/10.1002/pssb.2221710104.
H. Schmidt, G. Frohberg, and H. Wever, Acta Metall. Mater. 40, 3105 (1992). https://doi.org/10.1016/0956-7151(92)90473-R.
A. Paul, C. Ghosh, and W.J. Boettinger, Metall. Mater. Trans. A 42A, 952 (2011). https://doi.org/10.1007/s11661-010-0592-9.
V.A. Baheti, S. Islam, P. Kumar, R. Ravi, R. Narayanan, D. Hongqun, V. Vuorinen, T. Laurila, and A. Paul, Phil. Mag. 96, 15 (2016). https://doi.org/10.1080/14786435.2015.1119905.
S.P. Gupta, and D. Rathor, Z. Metallkde. 93, 516 (2002). https://doi.org/10.3139/146.020516.
K.N. Tu, and R.D. Thompson, Acta Metall. 30, 947 (1982). https://doi.org/10.1016/0001-6160(82)90201-2.
M. Onishi, and H. Fujibuchi, Trans. Jap. Inst. Met. 16, 539 (1975). https://doi.org/10.2320/matertrans1960.16.539.
J. Görlich, G. Schmitz, and K.N. Tu, Appl. Phys. Lett. 86, 53106 (2005). https://doi.org/10.1063/1.1852724.
J. Hektor, M. Ristinmaa, H. Hallberg, S.A. Hall, and S. Iyengar, Acta Mater. 108, 98 (2016). https://doi.org/10.1016/j.actamat.2016.02.016.
N. Zhao, Y. Zhong, M.L. Huang, H.T. Ma, and W. Dong, Sci. Rep. 5, 13491 (2015). https://doi.org/10.1038/srep13491.
Z.H. Zhang, M.Y. Li, Z.Q. Liu, and S.H. Yang, Acta Mater. 104, 1 (2016). https://doi.org/10.1016/j.actamat.2015.11.034.
H. Wondratschek, and W. Jeitschko, Acta Crystallogr. A 32, 664 (1976). https://doi.org/10.1107/S056773947600137X.
C. Cayron, Acta Crystallogr. A 62, 21 (2006). https://doi.org/10.1107/S010876730503686X.
G. van Tendeloo, and S. Amelinckx, Acta Crystallogr. A 30, 431 (1974). https://doi.org/10.1107/S0567739474000933.
G. Ghosh, and M. Asta, J. Mater. Res. 20, 3102 (2005). https://doi.org/10.1557/JMR.2005.0371.
Z.H. Zhang, C.W. Wei, H.J. Cao, J.J. Han, and Y. Zhang, Materials 12, 4127 (2019). https://doi.org/10.3390/ma12244127.
Acknowledgment
The authors thank Dipl.-Ing. Diane Hübgen for her assistance in metallographic preparation.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leineweber, A., Löffler, M. & Martin, S. Stable and Metastable Phase Equilibria Involving the Cu6Sn5 Intermetallic. J. Electron. Mater. 50, 5898–5914 (2021). https://doi.org/10.1007/s11664-021-09067-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09067-4