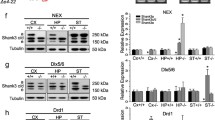
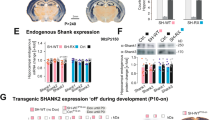
Abstract
Alterations in the balance between excitation and inhibition, especially in the brain’s critical developmental periods, are considered an integral part of the pathophysiology of autism. However, the precise mechanisms have not yet been established. SH3 and multiple Ankyrin repeat domains 3 (Shank3) deficient mice represent a well-established transgenic model of a neurodevelopmental disorder with autistic symptomatology. In this study, we characterize the consequences of Shank3 deficiency according to (1) expression of specific markers of different neuronal populations in pups and adult mice and (2) social behaviour and anxiety in adult mice. Our research found enhanced expression of serotonin transporter and choline acetyltransferase in the hippocampus and hypothalamus in Shank3-deficient pups. We demonstrated marked brain region differences in expression of excitatory glutamatergic markers in pups and adult Shank3 deficient mice. We also observed reduced expression of inhibitory GABAergic markers and GABA receptor subunits in several brain areas in both pups and adult Shank3 deficient mice. Further analysis of dopaminergic brain areas (nucleus accumbens, ventral tegmental area) revealed lower expression levels of GABAergic markers in adult Shank3 deficient mice. Adult Shank3− deficient mice exhibited excessive repetitive behaviour, a higher level of anxiety, and lower locomotor activity. Our data support the theory of an imbalance between excitatory and inhibitory neurotransmission in conditions of abnormal SHANK3 protein. We therefore suggest that autism-like conditions are accompanied by reduced expression of GABAergic markers in the brain during early development as well as in the adult age, which could be associated with long-lasting behavioural abnormalities.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sun X, Allison C, Auyeung B, Zhang Z, Matthews FE, Baron-Cohen S, Brayne C (2015) Validation of existing diagnosis of autism in mainland China using standardised diagnostic instruments. Autism 19(8):1010–1017. doi:https://doi.org/10.1177/1362361314556785
Sengupta P (2013) The laboratory rat: relating its age with human’s. Int J Prev Med 4(6):624–630
Heavner WE, Smith SEP (2020) Resolving the synaptic versus developmental dichotomy of autism risk genes. Trends Neurosci 43(4):227–241. https://doi.org/10.1016/j.tins.2020.01.009
Morton RA, Yanagawa Y, Valenzuela CF (2016) Electrophysiological assessment of serotonin and GABA neuron function in the dorsal raphe during the third trimester equivalent developmental period in mice. eNeuro. https://doi.org/10.1523/ENEURO.0079-15.2015
Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, Fishell G (2016) Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron 89(3):521–535. https://doi.org/10.1016/j.neuron.2015.11.020
Zander JF, Münster-Wandowski A, Brunk I, Pahner I, Gómez-Lira G, Heinemann U, Gutiérrez R, Laube G, Ahnert-Hilger G (2010) Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci 30(22):7634–7645. doi:https://doi.org/10.1523/JNEUROSCI.0141-10.2010
Nelson SB, Valakh V (2015) Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87(4):684–698. https://doi.org/10.1016/j.neuron.2015.07.033
Bruining H, Hardstone R, Juarez-Martinez EL, Sprengers J, Avramiea AE, Simpraga S, Houtman SJ, Poil SS, Dallares E, Palva S, Oranje B, Matias Palva J, Mansvelder HD, Linkenkaer-Hansen K (2020) Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics. Sci Rep 10(1):9195. doi:https://doi.org/10.1038/s41598-020-65500-4
Burrows EL, Koyama L, May C, Hill-Yardin EL, Hannan AJ (2020) Environmental enrichment modulates affiliative and aggressive social behaviour in the neuroligin-3 R451C mouse model of autism spectrum disorder. Pharmacol Biochem Behav 195:172955. https://doi.org/10.1016/j.pbb.2020.172955
Gąssowska-Dobrowolska M, Cieślik M, Czapski GA, Jęśko H, Frontczak-Baniewicz M, Gewartowska M, Dominiak A, Polowy R, Filipkowski RK, Babiec L, Adamczyk A (2020) Prenatal exposure to valproic acid affects microglia and synaptic ultrastructure in a brain-region-specific manner in young-adult male rats: relevance to autism spectrum disorders. Int J Mol Sci 21(10):3576. https://doi.org/10.3390/ijms21103576
Jaramillo TC, Xuan Z, Reimers JM, Escamilla CO, Liu S, Powell CM (2020) Early restoration of Shank3 expression in Shank3 knock-out mice prevents core ASD-like behavioral phenotypes. eNeuro. https://doi.org/10.1523/ENEURO.0332-19.2020
Castelhano AS, CassaneGdos S, Scorza FA, Cysneiros RM (2013) Altered anxiety-related and abnormal social behaviors in rats exposed to early life seizures. Front Behav Neurosci 7:36. https://doi.org/10.3389/fnbeh.2013.00036
Bögi E, Belovičová K, Moravčíková L, Csatlósová K, Dremencov E, Lacinova L, Dubovicky M (2019) Pre-gestational stress impacts excitability of hippocampal cells in vitro and is associated with neurobehavioral alterations during adulthood. Behav Brain Res 375:112131. doi:https://doi.org/10.1016/j.bbr.2019.112131
Wang J, Fernández AE, Tiano S, Huang J, Floyd E, Poulev A, Ribnicky D, Pasinetti GM (2018) An extract of Artemisia dracunculus L. promotes psychological resilience in a mouse model of depression. Oxid Med Cell Longev 2018:7418681. https://doi.org/10.1155/2018/7418681
Du Z, Tertrais M, Courtand G, Leste-Lasserre T, Cardoit L, Masmejean F, Halgand C, Cho YH, Garret M (2017) Differential alteration in expression of striatal GABAAR subunits in mouse models of huntington’s disease. Front Mol Neurosci 10:198. https://doi.org/10.3389/fnmol.2017.00198
Lopatina OL, Malinovskaya NA, Komleva YK, Gorina YV, Shuvaev AN, Olovyannikova RY, Belozor OS, Belova OA, Higashida H, Salmina AB (2019) Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev Neurosci 30(8):807–820. doi:https://doi.org/10.1515/revneuro-2019-0014
Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsäter H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Rogé B, Héron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39(1):25–27. doi:https://doi.org/10.1038/ng1933
Guilmatre A, Huguet G, Delorme R, Bourgeron T (2014) The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 74(2):113–122. doi:https://doi.org/10.1002/dneu.22128
Angelakos CC, Tudor JC, Ferri SL, Jongens TA, Abel T (2019) Home-cage hypoactivity in mouse genetic models of autism spectrum disorder. Neurobiol Learn Mem 165:107000. doi:https://doi.org/10.1016/j.nlm.2019.02.010
Monteiro P, Feng G (2017) SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci 18(3):147–157. https://doi.org/10.1038/nrn.2016.183
Qiu S, Li Y, Li Y, Zhong W, Shi M, Zhao Q, Zhang K, Wang Y, Lu M, Zhu X, Jiang H, Yu Y, Cheng Y, Liu Y (2018) Association between SHANK3 polymorphisms and susceptibility to autism spectrum disorder. Gene 651:100–105. https://doi.org/10.1016/j.gene.2018.01.078
Cope EC, Briones BA, Brockett AT, Martinez S, Vigneron PA, Opendak M, Wang SS, Gould E (2016) Immature neurons and radial glia, but not astrocytes or microglia, are altered in adult Cntnap2 and Shank3 mice, models of autism. eNeuro. https://doi.org/10.1523/ENEURO.0196-16.2016
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, Kheirbek MA (2018) Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97(3):670-683.e6. https://doi.org/10.1016/j.neuron.2018.01.016
Ko J (2017) Neuroanatomical substrates of rodent social behavior: the medial prefrontal cortex and Its projection patterns. Front Neural Circuits 11:41. https://doi.org/10.3389/fncir.2017.00041
Bissonette GB, Roesch MR (2016) Development and function of the midbrain dopamine system: what we know and what we need to. Genes Brain Behav 15(1):62–73. https://doi.org/10.1111/gbb.12257
Bey AL, Wang X, Yan H, Kim N, Passman RL, Yang Y, Cao X, Towers AJ, Hulbert SW, Duffney LJ, Gaidis E, Rodriguiz RM, Wetsel WC, Yin HH, Jiang YH (2018) Brain region-specific disruption of Shank3 in mice reveals a dissociation for cortical and striatal circuits in autism-related behaviors. Transl Psychiatry 8(1):94. doi:https://doi.org/10.1038/s41398-018-0142-6
Chen Q, Deister CA, Gao X, Guo B, Lynn-Jones T, Chen N, Wells MF, Liu R, Goard MJ, Dimidschstein J, Feng S, Shi Y, Liao W, Lu Z, Fishell G, Moore CI, Feng G (2020) Dysfunction of cortical GABAergic neurons leads to sensory hyper-reactivity in a Shank3 mouse model of ASD. Nat Neurosci 23(4):520–532. doi:https://doi.org/10.1038/s41593-020-0598-6
Palkovits M (1973) Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res 59:449–450. doi:https://doi.org/10.1016/0006-8993(73)90290-4
Palkovits M, Brownstein M (1988) Maps and guide to microdissection of the rat brain. Elsevier, New York
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, New York
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. doi:https://doi.org/10.1006/meth.2001.1262
Havranek T, Zatkova M, Lestanova Z, Bacova Z, Mravec B, Hodosy J, Strbak V, Bakos J (2015) Intracerebroventricular oxytocin administration in rats enhances object recognition and increases expression of neurotrophins, microtubule-associated protein 2, and synapsin I. J Neurosci Res 93(6):893–901. doi:https://doi.org/10.1002/jnr.23559
Seibenhener ML, Wooten MC (2015) Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 96:e52434. https://doi.org/10.3791/52434
Drapeau E, Riad M, Kajiwara Y, Buxbaum JD (2018) Behavioral phenotyping of an improved mouse model of Phelan-McDermid syndrome with a complete deletion of the Shank3 sene. eNeuro. https://doi.org/10.1523/ENEURO.0046-18.2018
Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR (2011) Assessment of social interaction behaviors. J Vis Exp 48:2473. doi:https://doi.org/10.3791/2473
Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN (2004) Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 3(5):303–314. doi:https://doi.org/10.1111/j.1601-183X.2004.00071.x
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. doi:https://doi.org/10.1038/nprot.2006.116
Reichova A, Bacova Z, Bukatova S, Kokavcova M, Meliskova V, Frimmel K, Ostatnikova D, Bakos J (2020) Abnormal neuronal morphology and altered synaptic proteins are restored by oxytocin in autism-related SHANK3 deficient model. Mol Cell Endocrinol 518:110924. doi:https://doi.org/10.1016/j.mce.2020.110924
Michalski D, Keck AL, Grosche J, Martens H, Härtig W (2018) Immunosignals of oligodendrocyte markers and myelin-associated proteins are critically affected after experimental stroke in wild-type and Alzheimer modeling mice of different ages. Front Cell Neurosci 12:23. https://doi.org/10.3389/fncel.2018.00023
Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJ, Trezza V (2016) Dopaminergic neurotransmission in the nucleus accumbens modulates social play behavior in rats. Neuropsychopharmacology 41(9):2215–2223. https://doi.org/10.1038/npp.2016.22
Pearson BL, Corley MJ, Vasconcellos A, Blanchard DC, Blanchard RJ (2013) Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behav Brain Res 243:138–145. doi:https://doi.org/10.1016/j.bbr.2012.12.062
Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K (2011) Neuron number and size in prefrontal cortex of children with autism. JAMA 306(18):2001–2010. doi:https://doi.org/10.1001/jama.2011.1638
Yang CJ, Tan HP, Du YJ (2014) The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience 267:1–10. doi:https://doi.org/10.1016/j.neuroscience.2014.02.021
Muller CL, Anacker AMJ, Veenstra-VanderWeele J (2016) The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 321:24–41. https://doi.org/10.1016/j.neuroscience.2015.11.010
Siemann JK, Muller CL, Forsberg CG, Blakely RD, Veenstra-VanderWeele J, Wallace MT (2017) An autism-associated serotonin transporter variant disrupts multisensory processing. Transl Psychiatry 7(3):e1067. doi:https://doi.org/10.1038/tp.2017.17
Filice F, Vörckel KJ, Sungur A, Wöhr M, Schwaller B (2016) Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol Brain 9:10. doi:https://doi.org/10.1186/s13041-016-0192-8
Lee B, Zhang Y, Kim Y, Kim S, Lee Y, Han K (2017) Age-dependent decrease of GAD65/67 mRNAs but normal densities of GABAergic interneurons in the brain regions of Shank3-overexpressing manic mouse model. Neurosci Lett 649:48–54. doi:https://doi.org/10.1016/j.neulet.2017.04.016
Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T (2005) GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47(6):803–815. doi:https://doi.org/10.1016/j.neuron.2005.08.023
Catavero C, Bao H, Song J (2018) Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res 371(1):33–46. doi:https://doi.org/10.1007/s00441-017-2668-y
Leonzino M, Busnelli M, Antonucci F, Verderio C, Mazzanti M, Chini B (2016) The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Rep 15(1):96–103. https://doi.org/10.1016/j.celrep.2016.03.013
Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN (2012) Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci 32(19):6525–6541. doi:https://doi.org/10.1523/JNEUROSCI.6107-11.2012
Yoo T, Cho H, Lee J, Park H, Yoo YE, Yang E, Kim JY, Kim H, Kim E (2018) GABA neuronal deletion of Shank3 exons 14–16 in mice suppresses striatal excitatory synaptic input and induces social and locomotor abnormalities. Front Cell Neurosci 12:341. https://doi.org/10.3389/fncel.2018.00341
Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, Liu S, Jaramillo TC, Bangash M, Xiao B, Worley PF, Powell CM (2013) Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci 33(47):18448–18468. doi:https://doi.org/10.1523/JNEUROSCI.3017-13.2013
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G (2011) Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472(7344):437–442. https://doi.org/10.1038/nature09965
Acknowledgements
This study was funded by the Grant Agency of the Ministry of Education and the Slovak Academy of Sciences (VEGA 2/0155/20, VEGA 2/0148/21), as well as by the Slovak Research and Development Agency project (APVV-15-205 and APVV-15-0045). We would like to thank Michael Sabo for proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Conceptualization: JB; ZB; Investigation: SB; ER; AR; JF; AS; BM.; Resources: ER, DO; ZB, JB; Writing - Original Draft: SB; ER; JB; ZB; Writing - Review & Editing: SB, DO, JB; ZB; Funding Acquisition: DO; JB; ZB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bukatova, S., Renczes, E., Reichova, A. et al. Shank3 Deficiency is Associated With Altered Profile of Neurotransmission Markers in Pups and Adult Mice. Neurochem Res 46, 3342–3355 (2021). https://doi.org/10.1007/s11064-021-03435-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03435-6