Abstract
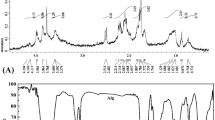
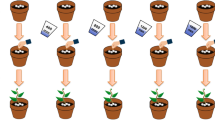
The objective of the study was to depolymerize alginate into short-length oligoalginates, adopting the simple solution plasma process (SPP) technique, for successful use in free radical scavenging and growth promotion in cell culture and agricultural practices. Alginate at various concentrations was depolymerized to oligoalginates using SPP by discharging for various times. The depolymerization into oligoalginates was proved by DNS, TLC, FT-IR, and HPAEC analyses and caused decrease in viscosity. Oligoalginates derived from 0.5% alginate (100 mg∙mL−1) showed the highest antioxidant activities in vitro. The oligoalginates enhanced growth of the human embryonic kidney (HEK293) cells to significant levels in a concentration-dependent manner without any extent of toxicity. The oligoalginates also promoted growth of lettuce. Thus, SPP is a powerful technique to break down alginate into oligoalginates that can be utilized as a free radical scavenger and as a growth promoter of animal cells and agricultural plants.








Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Gacesa, P. (1988). Alginates. A. Carbohydrate Polymers, 8, 161–182.
Liu, J., Yang, S., Li, X., Yan, Q., Reaney, M. J. T., & Jian, Z. (2019). Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Comprehensive Reviews in Food Science and Food Safety, 18, 1859–1881.
Lee, D.-W., Choi, W.-S., Byun, M.-W., Park, H.-J., Yu, Y.-M., & Lee, C.-M. (2003). Effect of γ-Irradiation on Degradation of Alginate. Journal of Agriculture and Food Chemistry, 51(16), 4819–4823.
Brownlee, I. A., Allen, A., Pearson, J. P., Dettmar, P. W., Havler, M. E., Atherton, M. R., & Onsoyen, E. (2005). Alginate as a source of dietary fiber. Critical Reviews in Food Science and Nutrition, 45, 497–510.
Murat, S., Rendevski, S., Akkas-Kavaklı, P., & Amir, S. (2010). Effect of G/M ratio on the radiation-induced degradation of sodium alginate. Radiation Physics and Chemistry, 79, 279–282.
Zhao, X., Li, B., Xue, C., & Sun, L. (2012). Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. Journal of Applied Phycology, 24, 295–300.
Kelishomi, Z. H., Goliaei, B., Mahdavi, H., Nikoofar, A., Rahimi, M., Movahedi, A. M., et al. (2016). Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chemistry, 196, 897–902.
Szekalska, M., Puciłowska, A., Szymańska, E., Ciosek, P., & Winnicka, K. (2016). Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. International Journal of Polymer Science, 2016. https://doi.org/10.1155/2016/7697031
Nagasawa, N., Mitomo, H., Yoshii, F., & Kume, T. (2000). Radiation-induced degradation of sodium alginate. Polymer Degradation and Stability, 69, 279–285.
Bolewski, K. (1967). Rate of ultrasonic degradation of sodium alginate in solutions of various ionic strengths. PolymerScience USSR, 9(2), 487–494.
Kim, H., Chung, J., Wang, D., Lee, J., Woo, H. C., Choi, I. G., & Kim, G. H. (2012). Depolymerization of alginate into a monomeric sugar acid using Alg17C, an exo-oligoalginatelyase cloned from Saccharophagus degradans. Applied Microbiology and Biotechnology, 93, 2233–2239.
MubarakAli, D., Kim, S. C., Lee, S., & Kim, J. (2015). One-step synthesis and characterization of broad spectrum antimicrobial efficacy of cellulose/ silver nanobiocomposites using solution plasma process. RSC Advance, (118), 1511–1517.
MubarakAli, D., Saravanakumar, K., Akbarsha, M. A., Lee, S., & Kim, J. (2019). Synthesis of Biocompatible Cellulose-Coated Nanoceria with pH-Dependent Antioxidant Property. ACS Applied Bio Materials, 2(5), 1792–1801.
Nam, S., MubarakAli, D., & Kim, J. (2016). Characterization of alginate/silver nanobiocomposites synthesized by solution plasma process and their antimicrobial properties. Journal of Nanomaterials. Article ID 4712813, 9 pages.
MubarakAli, D., Lee, S., & Kim, J. (2018). Solution plasma mediated formation of low molecular weight chitosan and its application as a biomaterial. International Journal of Biological Macromolecules, 118, 1511–1517.
Luan, Q., Ha, T. T., Uyen, P., & Hien, N. Q. (2012). Preparation of Oligoalginate Plant Growth Promoter by gamma Irradiation of Alginate Solution Containing Hydrogen Peroxide. Journal of Agricultural and Food Chemistry, 60(7), 737–741.
Rhein-Knudsen, N., Ale, M., & Meyer, A. (2015). Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Marine Drugs, 13, 3340–3359.
Khanra, S., Mondal, M., Halder, G., Tiwari, O. N., Gayen, K., & Bhowmick, T. K. (2018). Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food and Bioproducts Processing, 110, 60–84.
Miller, G. L. (1959). Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Analytical Chemistry, 31, 426–428.
Yang, Z., Li, J. P., & Guana, J. (2004). Preparation and characterization of oligomannuronates from alginate degraded by hydrogen peroxide. Carbohydrate Polymers, 58, 115–121.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–66.
MubarakAli, D., Manzoor, M. A., Sabarinathan, A., Devi, C. A., Rekha, P. D., Thajuddin, N., & Lee, S. Y. (2019). An investigation of antibiofilm and cytotoxic property of MgO nanoparticles. Biocatalysis and Agricultural Biotechnology, 18, 101069.
Cohen, I. R., Grassel, S., & Murdoch, A. D. (1993). Structural characterization of the complete human perlecan gene and its promoter. Proceedings of the National Academy of Sciences of the United States of America, 90, 10404–10408.
Lord, C., Rutter, M., DiLavore, P. C., Risi, S., Gotham, K., & Bishop, S. (2012). emopenAutism diagnostic observation schedule: ADOSemclose. Western Psychological Services: Los Angeles.
Watthanaphanit, A., & Saito, N. E. (2013). Effect of polymer concentration on the depolymerization of sodium alginate by the solution plasma process. Polymer Degradation and Stability, 98, 1072–1080.
Swift, S. M., Hudgens, J. W., Heselpoth, R. D., Bales, P. M., & Nelson, D. C. (2014). Characterization of AlgMsp, an Alginate Lyase from Microbulbifer sp. Plos One, 9(11), e112939.
Wang, X. T., Wang, L. L., Che, J., Li, X. Y., Li, J. G., Wang, J., & Xu, Y. P. (2014). Isolation of a novel alginate lyaseproducing Bacillus litoralis strain and its potential to ferment Sargassumhorneri for biofertilizer. Aquaculture, 434, 434–441.
Sakugawa, K., Ikeda, A., Takemura, A., & Ono, H. (2004). Simplified method for estimation of composition of alginates by FTIR. Journal of Applied Polymer Science, 93, 1372–1377.
Lawrie, G., Keen, I., Drew, B., Chandler-Temple, A., Rintoul, L., Fredericks, P., & Grøndahl, L. (2007). Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromole, 8, 2533–2541.
Salachna, P., Grzeszczuk, M., Meller, E., & Soból, M. (2018). Oligo-Alginate with Low Molecular Mass Improves Growth and Physiological Activity of Eucomisautumnalis under Salinity Stress. Molecules, 23, 812. https://doi.org/10.3390/molecules23040812.
Hernandez-Marin, E., & Martínez, A. (2012). Carbohydrates and Their Free Radical Scavenging Capability: A Theoretical Study. The Journal of Physical Chemistry B, 116(32), 9668–9675.
Falkeborg, M., Cheong, L. Z., Gianfico, C., Sztukiel, K. M., Kristensen, K., Glasius, M., & Guo, Z. (2014). Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chemistry, 164, 185–194.
Galateanu, B., Dimonie, D., Vasile, E., Nae, S., Cimpean, A., & Costache, M. (2012). Layer-shaped alginate hydrogels enhance the biological performance of human adipose-derived stem cells. BMC Biotechnology, 2(1), 35.
Yan, G. L., Guo, Y. M., Yuan, J. M., Liu, D., & Zhang, B. K. (2011). Sodium alginate oligosaccharides from brown algae inhibit Salmonella enteritidis colonization in broiler chickens. Poultry Science, 90, 1441–1448.
Acknowledgements
All the authors are thankful to Prof. M.A. Akbarsha, Emeritus Professor, National College (NCT), India, for proofreading the article.
Funding
This study was supported by the grant from Incheon National University, Republic of Korea, 2016.
Author information
Authors and Affiliations
Contributions
DM: data acquisition, writing manuscript; ML: data acquisition; MM: cytotoxicity assay; SL: review and proofreading; JK: review, guidance, and proofreading.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Clean and rapid production of oligoalginate through solution plasma process.
• Oligoalginates derived from 0.5% alginate (100 mg mL−1) promoted growth of lettuce.
• Interestingly, oligoalginate promoted the growth of HEK293 cells with no toxicity.
Rights and permissions
About this article
Cite this article
MubarakAli, D., Lee, M., Manzoor, M.A. et al. Production of Oligoalginate via Solution Plasma Process and Its Capability of Biological Growth Enhancement. Appl Biochem Biotechnol 193, 4097–4112 (2021). https://doi.org/10.1007/s12010-021-03640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03640-7