Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6879
Peer-review started: April 17, 2021
First decision: May 10, 2021
Revised: May 15, 2021
Accepted: June 28, 2021
Article in press: June 28, 2021
Published online: August 16, 2021
The majority of renal cell carcinomas are single lesions; unilateral synchronous multifocal renal carcinoma (USMRC) is rarely reported and poses a treatment challenge for urological oncologists.
A 56-year-old man was hospitalized for pain and discomfort in the right kidney area for 6 d. Contrast-enhanced computed tomography demonstrated cT1a renal tumors at the lower pole of the right kidney and a cT1b renal tumor at the middle dorsal portion of the right kidney. The patient underwent retroperitoneal laparoscopic partial nephrectomy (RLPN). There were no complications peri-opera
RLPN is a safe, effective, and feasible for the management of USMRC, which can obtain equivalent oncological results with optimal renal function preservation.
Core Tip: Unilateral synchronous multifocal renal carcinoma (USMRC) is defined as having more than two malignant tumors with a spacing ≥ 1 cm in one kidney. USMRC is rarely reported and nephron-sparing surgery for USMRC is difficult. We describe a patient with USMRC who underwent retroperitoneal laparoscopic partial nephrectomy (RLPN). There were no complications peri-operatively. Histopathology revealed clear cell renal cell carcinoma at the lower pole of the right kidney and chromophobe renal cell carcinoma at the middle dorsal portion of the right kidney. No tumor bed recurrence or metastasis was observed on imaging and his renal function remained stable during the 12-mo follow-up. Thus, RLPN is safe, effective, and feasible for the management of USMRC.
- Citation: Xiao YM, Yang SK, Wang Y, Mao D, Duan FL, Zhou SK. Retroperitoneal laparoscopic partial nephrectomy for unilateral synchronous multifocal renal carcinoma with different pathological types: A case report. World J Clin Cases 2021; 9(23): 6879-6885
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6879.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6879
Renal carcinoma is a common tumor of the urinary system, which accounted for 2.2% of new cancer cases and 1.8% of cancer deaths in the GLOBOCAN 2020 database[1]. Unilateral synchronous multifocal renal carcinoma (USMRC) is defined as having more than two malignant tumors with a spacing ≥ 1 cm in one kidney. USMRCs are rare and occur in less than 5% of all renal tumor patients[2]. Herein, we report a case of USMRC with different pathological types in the right kidney; the patient underwent retroperitoneal laparoscopic partial nephrectomy (RLPN).
A 56-year-old man was hospitalized for pain and discomfort in the right kidney area for 6 d.
At 6 d before admission, the patient had pain in the right kidney area. No history of trauma was reported. During these 6 d, the patient did not receive any treatment.
The patient had a history of asthma, which had been under medical control for more than 6 years. There was no history of hypertension, diabetes mellitus, coronary artery disease, or stroke.
He was a smoker for 20 years with an average of 15 cigarettes/d. No drinking history or hereditary family history was noted.
No obvious abnormalities were found on physical examination.
Serum laboratory testing and electrocardiography were normal. Preoperative examination indicated that serum creatinine was 63.7 μmol/L.
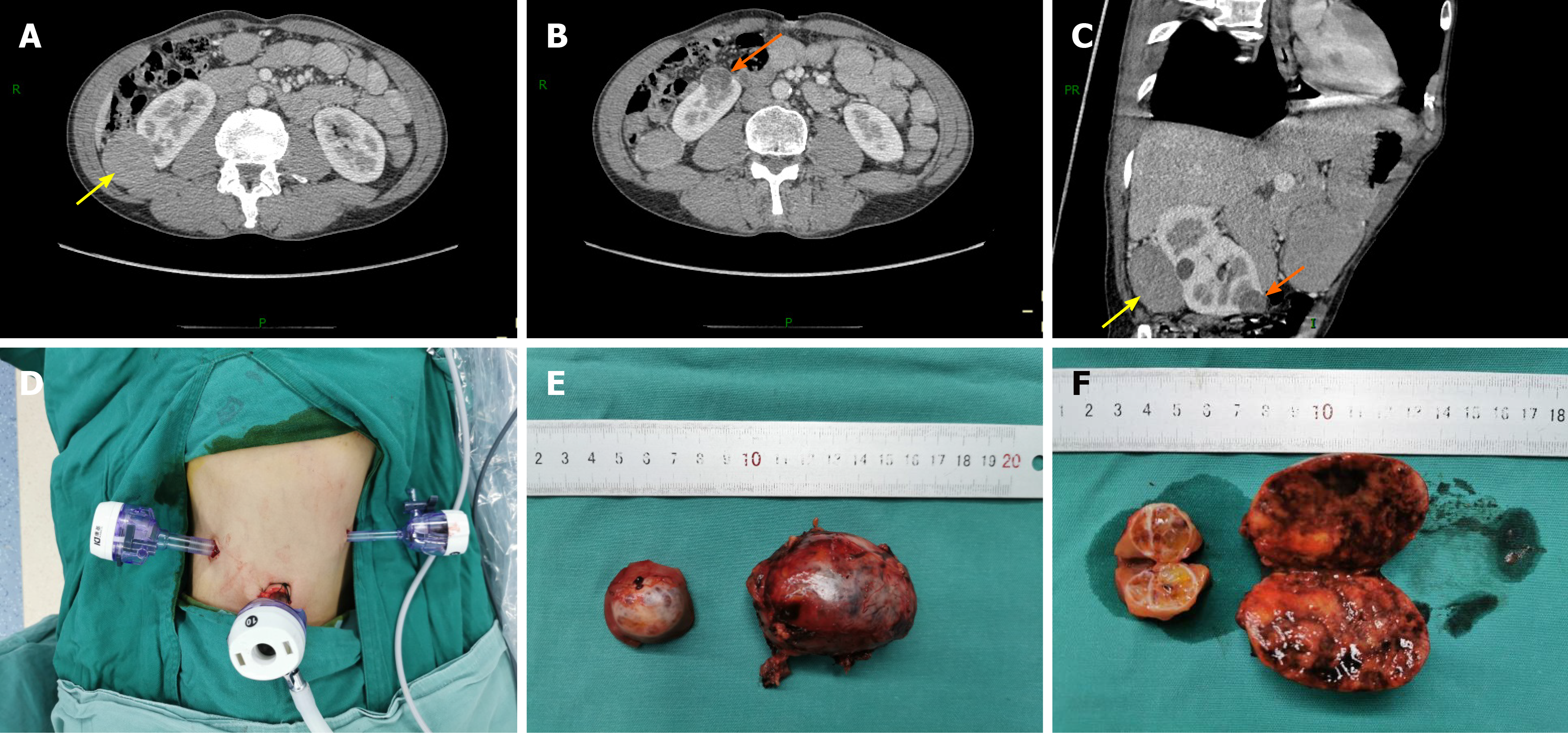
Contrast-enhanced computed tomography (CECT) of the abdomen showed soft tissue mass shadows with progressive enhancement in the space between the liver and kidney, approximately 1.6 cm × 4.0 cm in size, and a slight low-density nodular shadow with heterogeneous enhancement was seen at the lower pole of the right kidney, approximately 1.7 cm × 2.4 cm in size (Figure 1). The glomerular filtration rate (GFR), estimated by 99 mTc-DTPA dynamic renal imaging, was normal in both kidneys (left: 71.5 mL/min, right: 43.4 mL/min), and the total GFR (124.6 mL/min) was in the normal range.
The final diagnosis of the presented case was clear cell renal cell carcinoma at the lower pole of the right kidney and chromophobe renal cell carcinoma at the middle dorsal portion of the right kidney.
The patient underwent RLPN. He was placed in the right lateral position with his waist raised, the trocar shell was placed at the point under the 12th rib along the right posterior axillary line, the 11th rib pointed along the right axillary front, 2 cm above the iliac crest along the axillary midline, and trocars of 10, 10, and 5 mm were inserted at these three locations, respectively. A 12-15 mmHg pneumoperitoneum was created during surgery. The retroperitoneal fat along the psoas major was removed; the perirenal fascia was opened; and the renal artery was identified, separated, and then cleared of perirenal fat. The small tumor was found medial to the lower pole of the kidney, and the large tumor was located in the middle dorsal portion of the kidney. The tumor boundaries were determined, the renal artery was temporarily blocked using a bulldog clamp, and the renal tumors were completely removed with scissors along the 0.5 cm edge of the tumor. The tumor basement was relatively superficial. A tumor margin biopsy was taken for pathological examination and the renal incision was closed with 2-0 agnail stitches, after which the vascular blocking forceps were loosened and the renal artery opened. The kidney ischemia time was 27 min. Together, two tumors were resected during surgery (Figure 1). The operation was completed with a total blood loss of 80 mL, and the tumors were removed using a specimen bag.
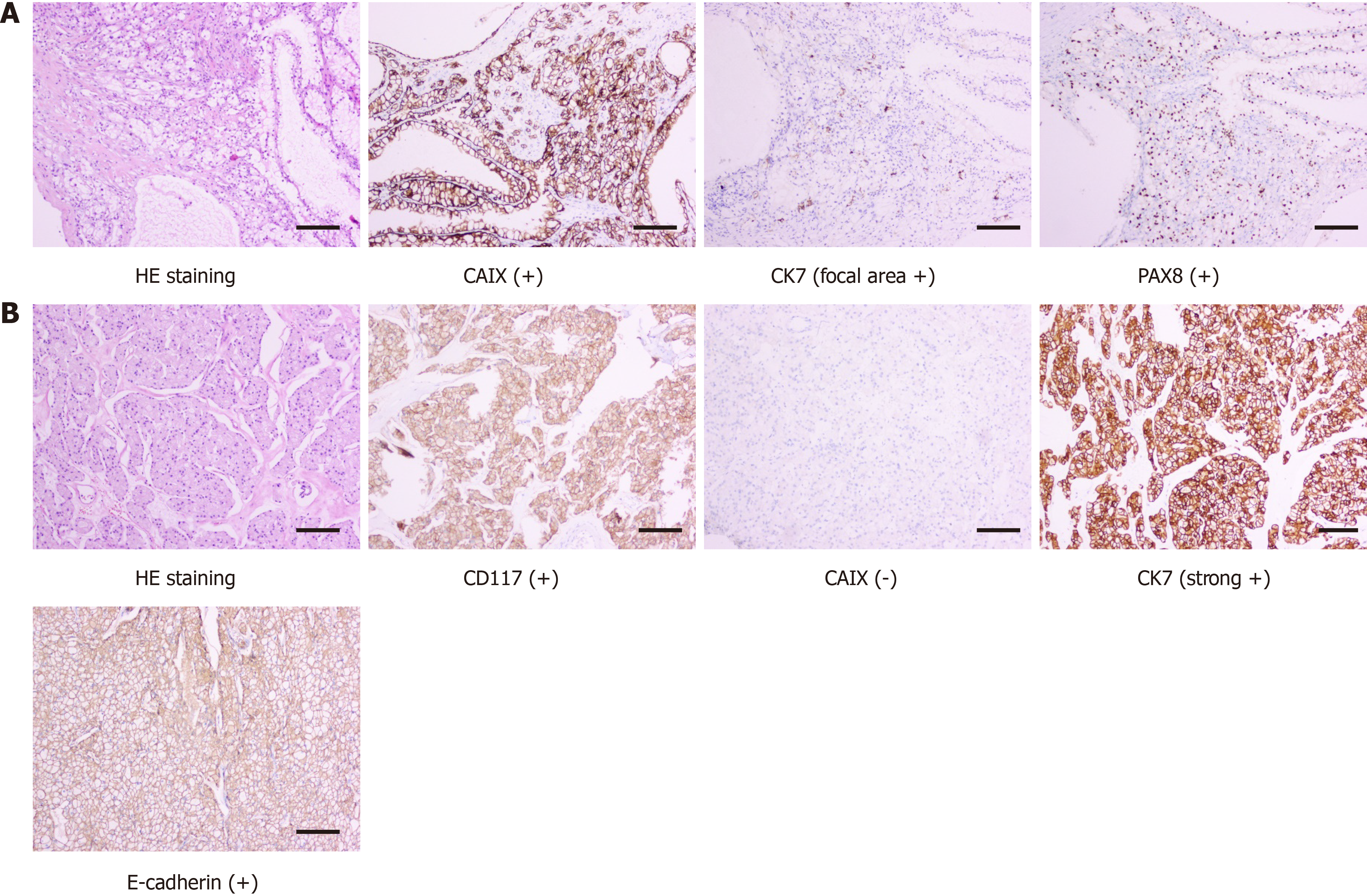
Histopathology, as confirmed by immunohistochemical studies, showed that the small right kidney tumor was a clear cell tumor, with a multilocular cystic area, most of the area was covered with simple transparent cells, and a focal area of solid tumor cell nests was observed. The tumor cell immune phenotype consisted of paired box gene 8 (PAX8)(+), cytokeratin 7 (CK7, focal area +), cluster of differentiation 10 (CD10, focal area +), carbonic anhydrase IX (CAIX) (+), vimentin (+), CK (+), Ki67 (+, 10%), transcription factor E3 (-), and succinate dehydrogenase complex iron sulfur subunit B (+). Combined with hematoxylin and eosin (H&E) staining and the immune phenotype, the tumor was considered to be renal clear cell carcinoma (ISUP/WHO Nuclear Grading, grade 1). The large right kidney tumor showed tumor cells with distinct cell membranes and a “granular” cytoplasm. Combined with H&E morphology and the tumor cell immune phenotype consisting of PAX8 (+), CK7 (strong +), E-cadherin (+), CD10 (focal area +), CK (+), epithelial membrane antigen (+), CD117 (+) CAIX (-), and Ki67 (+, 1%), the tumor was considered a chromophobe renal cell carcinoma (Figure 2). Surgical margins were negative and no gene mutations were found in the VHL gene test.
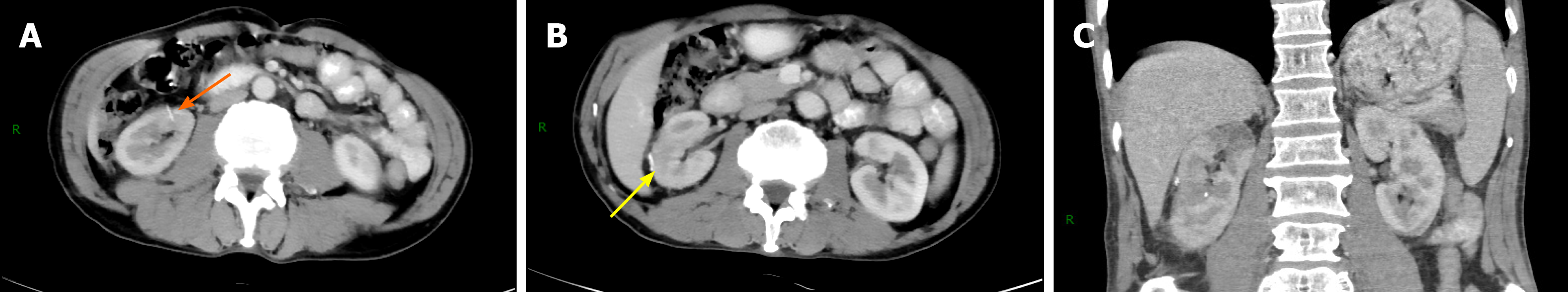
There was no additional decline in the serum creatinine value (65.2 μmol/L) and right kidney GFR value (38.9 mL/min) at 1 mo postoperatively. No tumor bed recurrence or metastasis was found on imaging (Figure 3), and the patient’s renal function remained stable during the 12-mo follow-up period.
Multiple tumors in a single kidney are rarely reported and most have been reported in small sample, single-center retrospective studies or case reports. USMRC should be actively treated with surgery, and the therapeutic principle is to completely remove the tumor and retain maximum renal function.
Radical nephrectomy (RN) has been traditionally used to treat patients with multifocal renal masses in an effort to maximize oncological benefit. However, RN results in renal unit loss, renal function decline, and reduced quality of life. Compared with partial nephrectomy (PN), RN obviously increases the risk of chronic kidney disease, which is associated with mortality, cardiovascular morbidity, and hospitalization[3,4]. For multiple tumors in a solitary kidney or contralateral kidney insufficiency, the clinical treatment is very difficult. Treatment of multiple tumors increases the time to tumor resection and incision suture, increases the time to renal warm ischemia, and increases the risk of intraoperative conversion from PN to RN. PN is currently recommended for pathologic stage T1 (pT1) renal cell carcinoma[5]. PN is relatively difficult for multiple lesion resection and renal reconstruction. For unilateral multifocal renal tumors, laparoscopic partial nephrectomy (LPN) has been proven safe and feasible with acceptable oncological results and complication rates in selected patients[6,7]. For USMRC cT1 stage, PN can prevent unnecessary renal unit damage and achieve the same effect as RN, and postoperative quality of life in patients is better[8-10]. For PN, there are three main goals known as the “trifecta”: a negative surgical margin, postoperative renal retention, and rapid postoperative recovery. The analysis by Yerram et al[11] indicated that both robotic and open PN can achieve the “trifecta” outcome for unilateral, synchronous, and multifocal renal tumors.
Currently, there are two approaches to perform LPN: transabdominal laparoscopic partial nephrectomy (TLPN) and RLPN. During TLPN, there is more surgical space to observe the anatomical marks, perform large tumor resection, and manage injuries that occur during surgery[12]. Nevertheless, the transabdominal approach may also cause complications during the separation of abdominal organs, including intestinal obstruction and intestinal paralysis. Furthermore, tumor exposure takes a longer time, and both eating and postoperative recovery can be disturbed to varying degrees. During RLPN, there is less difficulty in dissociating renal arteries and veins, and relatively less tissue separation, which can avoid damage to abdominal organs[13]. At the same time, it can also effectively prevent tumor cell seeding and abdominal contamination. For a dorsal kidney tumor, there is no need for extensive renal turnover through the retroperitoneal approach, which reduces the possibility of renal vein and ureter injury[14]. However, the surgical field during RLPN is narrow, and anatomical marks are not very obvious. Maximum tumor diameter and renal parenchyma invasion depth are the most accurate anatomical features and are predictors of nephrectomy type[15]. In this case, from CECT imaging measurement, the maximum diameter of the tumors was 5.3 cm (cT1b, TNM classification), and the maximal invasion depth in the lower pole and middle dorsal portion of the renal tumor was 1.5 and 0.8 cm, respectively. PN is performed when tumor invasion depth is less than 2.5 cm, whereas RN is performed when tumor invasion depth is more than 3.0 cm[15]. Thus, we selected RLPN as the operative method following preoperative CECT. No surgical complications were observed and the surgical margins were negative in this case. If the tumor is large (tumor diameter > 7 cm) or a completely intrarenal type, RN can be performed. Both the transabdominal and retroperitoneal approaches are safe and effective for the treatment of USMRC. The choice of surgical approach mainly depends on the characteristics of the renal tumor (e.g., tumor size, number and location), the operator’s habits and relevant surgical experience. In China, the retroperitoneal approach is usually adopted, while the transabdominal route is often chosen in Western countries.
The independent risk factors influencing postoperative renal function are renal retention volume and intraoperative renal ischemia time[16]. The kidney can withstand an ischemia time of approximately 30 min at room temperature, and irreversible renal function loss may occur after 30 min[17,18]. Intraoperative renal ischemia time is extended with increased tumor number and size or when the tumor is intrarenal. Renal ischemia time also has a significant effect on the recovery of postoperative renal function; thus, expert laparoscopic suture techniques play a key role in reducing ischemia time. Our experience revealed that for young and healthy patients, the kidney can tolerate a longer renal ischemia time. In contrast, for elderly patients and those with cardiovascular disease or renal dysfunction, renal ischemia time should be strictly controlled within 30 min. Our patient underwent RLPN with renal artery occlusion and the ischemia time was 27 min. Compared with preoperative right GFR values, there was no significant change in renal function 1 mo after the operation.
Tumor pathological types are related to tumor grade, metastasis and prognosis. The pathological results can also provide guidance for patient counseling and treatment planning. However, the pre-operative imaging technique did not accurately determine the tumor histology features, and postoperative pathological examination is still needed to confirm the diagnosis. For multiple synchronous renal tumors (unilateral and bilateral), age at diagnosis < 60 years, bilateral lesions and ≥ 3 tumors are predictive factors of histological concordance[19]. Sporadic bilateral synchronous renal tumors (BSRT) have a high pathological concordance. Patel et al[20] reported high malignant concordance rates in 89% (222/249) of patients with BSRT. Analysis of data from the SEER database demonstrated that the histologic concordance rate of BSRT patients reached 93% (256/274)[21]. However, although most USMRCs belong to the same pathological type in the literature, the pathological concordance rate of USMRC is relatively lower than that of BSRT. Simhan et al[2] reported that the pathological concordance of unilateral multifocal malignant renal tumors was observed in 77.2% and the most common pathological type was clear cell carcinoma (36.1%). Blute et al[22] showed that 59% (70/118) of patients with USMRC were concordant in histological subtype, and there were at least two histological malignancies with clear cell and papillary types most common in 17% of cases (20/118). In our case, histopathology revealed a low-grade, pT1a, clear-cell renal cell carcinoma at the lower pole of the right kidney and a pT1b, chromophobe renal cell carcinoma at the middle dorsal portion of the right kidney. It is worth noting that different pathological types does not affect the choice of surgical procedure (PN or RN), the latter depends on tumor size, location, depth, stage, and the surgeon’s skill level.
Our case represents a rare USMRC with different pathological types. A detailed preoperative evaluation and an appropriate operation are the key to surgical treatment. The retroperitoneal laparoscopic procedure is the preferred treatment for USMRC with a low complication rate, and ensures oncologic control and preserves renal function.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ho CM, Marickar F S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX
| 1. | Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 350] [Article Influence: 87.5] [Reference Citation Analysis (1)] |
| 2. | Simhan J, Canter DJ, Sterious SN, Smaldone MC, Tsai KJ, Li T, Viterbo R, Chen DY, Greenberg RE, Kutikov A, Uzzo RG. Pathological concordance and surgical outcomes of sporadic synchronous unilateral multifocal renal masses treated with partial nephrectomy. J Urol. 2013;189:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7995] [Cited by in F6Publishing: 8094] [Article Influence: 404.7] [Reference Citation Analysis (0)] |
| 4. | Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy vs radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55-61; discussion 61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 577] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 5. | Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, Cindolo L, Han KR, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Chopin DK, Figlin RA, Mulders PF, Belldegrun AS. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181-2185, quiz 2435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 415] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Mercimek MN, Ozbek LM, Ozden E. Unilateral Synchronous Multiple Kidney Tumors Managed by Laparoscopic Partial Nephrectomy: Five-year Follow-up. J Coll Physicians Surg Pak. 2019;29:S157-S159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Tsivian A, Tsivian M, Benjamin S, Sidi AA. Laparoscopic partial nephrectomy for multiple tumours: feasibility and analysis of peri-operative outcomes. BJU Int. 2011;108:1330-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Gupta GN, Peterson J, Thakore KN, Pinto PA, Linehan WM, Bratslavsky G. Oncological outcomes of partial nephrectomy for multifocal renal cell carcinoma greater than 4 cm. J Urol. 2010;184:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Krambeck A, Iwaszko M, Leibovich B, Cheville J, Frank I, Blute M. Long-term outcome of multiple ipsilateral renal tumours found at the time of planned nephron-sparing surgery. BJU Int. 2008;101:1375-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mano R, Kent M, Larish Y, Winer AG, Chevinsky MS, Hakimi AA, Sternberg IA, Sjoberg DD, Russo P. Partial and Radical Nephrectomy for Unilateral Synchronous Multifocal Renal Cortical Tumors. Urology. 2015;85:1404-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Yerram NK, Dagenais J, Bryk DJ, Nandanan N, Maurice MJ, Mouracade P, Kara O, Kaouk JH. Trifecta Outcomes in Multifocal Tumors: A Comparison Between Robotic and Open Partial Nephrectomy. J Endourol. 2018;32:615-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Tan M, Xu Y, Xu D, Jiang J, Zhao W, Cui D, Ruan Y, Xia S. Laparoscopic Partial Nephrectomy With Sequential Precise Tumor-specific Segmental Renal Artery Clamping for Multiple Ipsilateral Renal Tumors: A New Treatment Approach and Initial Experience. Urology. 2017;108:102-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Zhu J, Jiang F, Li P, Shao P, Liang C, Xu A, Miao C, Qin C, Wang Z, Yin C. Application and analysis of retroperitoneal laparoscopic partial nephrectomy with sequential segmental renal artery clamping for patients with multiple renal tumor: initial experience. BMC Urol. 2017;17:82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Wang B, Gong H, Zhang X, Li H, Ma X, Song E, Gao J, Dong J. Bilateral Synchronous Sporadic Renal Cell Carcinoma: Retroperitoneoscopic Strategies and Intermediate Outcomes of 60 Patients. PLoS One. 2016;11:e0154578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Tornberg SV, Kilpeläinen TP, Järvinen P, Visapää H, Järvinen R, Taari K, Nisén H. Renal Tumor Invasion Depth and Diameter are the Two Most Accurate Anatomical Features Regarding the Choice of Radical Versus Partial Nephrectomy. Scand J Surg. 2018;107:54-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, Novick AC. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008;180:2363-8; discussion 2368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 17. | Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005;95:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Porpiglia F, Renard J, Billia M, Musso F, Volpe A, Burruni R, Terrone C, Colla L, Piccoli G, Podio V, Scarpa RM. Is renal warm ischemia over 30 minutes during laparoscopic partial nephrectomy possible? Eur Urol. 2007;52:1170-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Beaugerie A, Audenet F, Verkarre V, Delavaud C, Le Guilchet T, Hurel S, de Saint Aubert N, Correas JM, Fontaine E, Richard S, Méjean A, Timsit MO. Pathological heterogeneity in sporadic synchronous renal tumors: Is the histological concordance predictable? Urol Oncol. 2018;36:11.e7-11.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Patel AR, Lee BH, Campbell SC, Zhou M, Fergany AF. Bilateral synchronous sporadic renal tumors: pathologic concordance and clinical implications. Urology. 2011;78:1095-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Rothman J, Crispen PL, Wong YN, Al-Saleem T, Fox E, Uzzo RG. Pathologic concordance of sporadic synchronous bilateral renal masses. Urology. 2008;72:138-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Blute M, Thibault GP, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Multiple ipsilateral renal tumors discovered at planned nephron sparing surgery: importance of tumor histology and risk of metachronous recurrence. J Urol. 2003;170:760-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |