Published online Aug 16, 2021. doi: 10.12998/wjcc.v9.i23.6858
Peer-review started: March 18, 2021
First decision: May 11, 2021
Revised: May 24, 2021
Accepted: June 15, 2021
Article in press: June 15, 2021
Published online: August 16, 2021
Forkhead box protein 1 (FOXP1) (OMIM: 605515) at chromosomal region 3p14.1 plays an important regulatory role in cell development and functions by regula
A 5-year-old boy mainly presented with attention deficit and hyperactivity disorder and developmental retardation accompanied by gait instability and abnormal facial features (low-set ears). DNA samples were extracted from the child’s and his parents’ peripheral blood to detect whole-exome sequences and whole-genome copy number variations. Results revealed heterozygous deletions of exon 6-21 of FOXP1 gene in the child. Physical examination upon admission showed that the child was generally in good condition, had a moderate nutritional status, a slightly slow response to external stimuli, equally large and equally round bilateral pupils, was sensitive to light reflection, and had poor eye contact and joint attention. He had no meaningful utterance and could not pronounce words properly. He was able to use gestures to simply express his thoughts, to perform simple actions, and to listen to instructions. He had no rash, cafe-au-lait macules, or depigmentation spots. He had thick black hair and low-set ears. He had highly sensitive skin, especially on his face and palms. He had no abnormal palm fingerprint. Cardiopulmonary and abdominal examinations revealed no abnormalities. He had normal limb muscle strength and tension. He showed normal tendon reflexes of both knees. His bilateral Babinski and meningeal irritation signs were negative. He had a normal male vulva.
We report the characteristic features of autism with dysphasia accompanied by mental retardation caused by FOXP1 exon deletion. This study provides a molecular basis for etiological diagnosis and treatment of the child, as well as for genetic counseling for the pedigree.
Core Tip: We report the characteristic features of autism with dysphasia accompanied by mental retardation caused by FOXP1 exon deletion. In this case, the FOXP1 gene of the child had a heterozygous deletion variation, and the mutation site was heterozygous deletion in exon 6-21. This study provides a molecular basis for etiological diagnosis and treatment of the child, as well as for genetic counseling for the pedigree.
- Citation: Lin SZ, Zhou XY, Wang WQ, Jiang K. Autism with dysphasia accompanied by mental retardation caused by FOXP1 exon deletion: A case report. World J Clin Cases 2021; 9(23): 6858-6866
- URL: https://www.wjgnet.com/2307-8960/full/v9/i23/6858.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i23.6858
Mental retardation accompanied by dysphasia in the absence or presence of autism (OMIM: 613670) is a developmental disease that manifests as mild to moderate mental retardation, dysphasia, and autism. This disease is clinically manifested by systemic developmental retardation, delay in walking and language, and behavior disorders including irritability, attention deficit and hyperactivity disorder (ADHD), aggression and stiff behavior. The occurrence of this disease is mainly affected by various factors such as heredity, immunity, and history of medications during pregnancy. Clinically, there are no specific drugs to treat this disease, and the normal physiological functions of a child can be primarily improved with combined training.
Forkhead box protein 1 (FOXP1) (OMIM: 605515) at chromosomal region 3p14.1 plays an important regulatory role in cell development and functions by regulating genetic expression. FOXP1, an oncogene, is capable of initiating tumorigenicity depending on the cell type[1-3]. FOXP1 also plays an important role in regulating the cell develop
A 5-year-old boy presented mainly with ADHD and developmental retardation accompanied by gait instability and abnormal facial features (low-set ears). DNA samples were extracted from the child’s and his parents’ peripheral blood to detect whole-exome sequences and whole-genome copy number variations (CNVs). The results revealed heterozygous deletions of exon 6-21 of FOXP1 gene in the child.
In 2018, a 3-year-old boy presented to our hospital with a 3-year history of developmental retardation. Since birth, the child had lagged behind in development; he had progressive improvements, could not pronounce words properly, could not utter any meaningful sentences, had symptoms of ADHD, and could perform only simple actions. The child learned to sit when he was aged approximately 9 mo. He could crawl when he was 1-year-old and only learned to walk independently when he was 2-years-old. He was admitted to our hospital for etiological diagnosis and treatment. Now, the child has ADHD, developmental retardation, gait instability, and low-set ears.
The child was his parents’ first. He was delivered by cesarean section at term, with a birth weight of 3.35 kg and a body length of 50 cm. He had no birth trauma or puerperal tetany, but he had a history of amniotic infection and jaundice for 1 mo. No obvious reason could be proposed for his developmental retardation with progressive improvements. The child had a normal diet without preferences. Hydrocephalus and atrial septal defect were revealed during prenatal examination at 32 wk.
The parents were in good health, and they denied consanguineous marriage and a history of familial inherited diseases.
Physical examination upon admission showed that the child was generally in good condition, had a moderate nutritional status, a slightly slow response to external stimuli, equally large and equally round bilateral pupils, was sensitive to light reflection, and had poor eye contact and joint attention. He had no meaningful utterance and could not pronounce words properly. He was able to use gestures to simply express his thoughts, to perform simple actions, and to listen to instructions. He had no rash, cafe-au-lait macules, or depigmentation spots. He had thick black hair and low-set ears. He had highly sensitive skin, especially on his face and palms. He had no abnormal palm fingerprint. Cardiopulmonary and abdominal examinations revealed no abnormalities. He had normal limb muscle strength and tension. He showed normal tendon reflexes of both knees. His bilateral Babinski and meningeal irritation signs were negative. He had a normal male vulva.
He exhibited restricted interest in common things and even had imaginary play with toys as a substitute for real things. He was capable of sitting and walking independently. He could pinch small pills with his thumb and forefinger and unscrew bottle caps. He could doodle with a pen in hand and build three layers of blocks. He showed uncoordinated walking movements. He was observed to easily fall. He was incapable of running, jumping.
Auxiliary examinations were conducted. Head magnetic resonance imaging on September 3, 2018 revealed slight dilatation of the right ventricle, with inborn anomalies considered. Vacuole turcica electrocardiography showed trace tricuspid regurgitation (with physiological considerations).
Cardiac function and electroencephalography (EEG) found no abnormalities.
Before treatment, the patient was rated according to the Autism Behavior Checklist (ABC), Gesell Developmental Schedules, and S-S Language Delay Assessment. He had an ABC score of 67 points on August 16, 2018. According to S-S Language Delay Assessment, the patient was categorized as Class I, with language delay, and the development level was about 2.0–2.5-years-old. According to Gesell Developmental Schedules, the patients had an adaptive development quotient of 44.13, with his language and personal–social skills severely lagging behind, and his other skills were moderately lagging behind.
The patient was preliminarily diagnosed with autism with dysphasia accompanied by mental retardation on the basis of his clinical manifestations and laboratory results. After regular rehabilitation treatment and functional exercises, his abilities in various fields developed: He could maintain eye contact; he could flexibly communicate every day using different sentence patterns; he could clearly articulate every note, although his speech was a little slow; he could perform two-step actions; and he could play simple drama games with adults familiar to him to reproduce life scenes, as well as perform parallel plays with peers without understanding the rules of the games. He is now capable of running, jumping, alternating his feet while going up and down the stairs, ride a scooter, build nine layers of blocks, and imitate circles, squares and crosses.
In 2020, whole-exome sequencing (WES) and whole-genome sequencing analysis of CNVs were performed at our hospital to seek genetic counseling and clarify the etiology of the child’s condition.
With the consent of the child’s guardian, 2 mL peripheral blood was extracted for genomic DNA analysis. WES and whole-genome sequencing analysis of CNVs were done by MGExome. The distribution frequency of mutation sites of the genetic variations detected were referred to 1000 Genomes, ESP6500 (NHLBI Exome Sequencing Project), The Exome Aggregation Consortium (EXAC) and EXAC-EXAC data on about 4000 East Asians. The reported pathogenic loci were confirmed by referring to the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk) and the Online Mendelian Inheritance in Man (http://omim.org). The pathogenicity of the variants was evaluated in accordance with the Guideline for Interpretation of Sequence Variants[6] published by the American College of Medical Genetics and Genomics (ACMG).
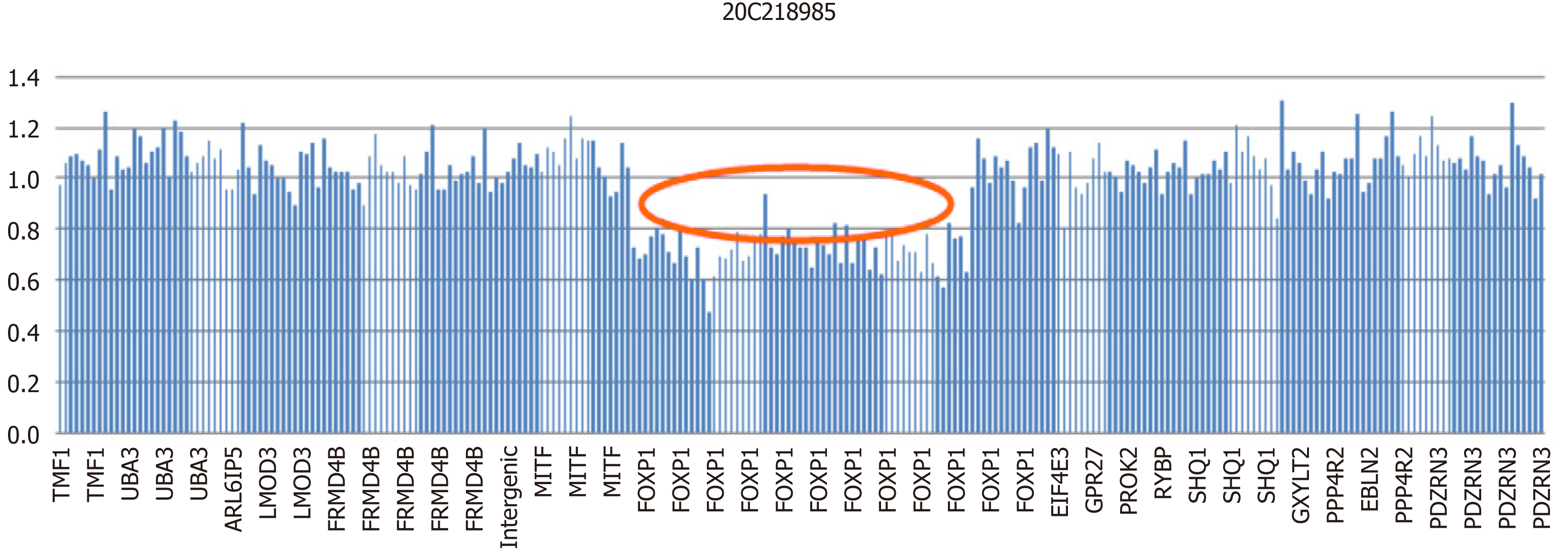
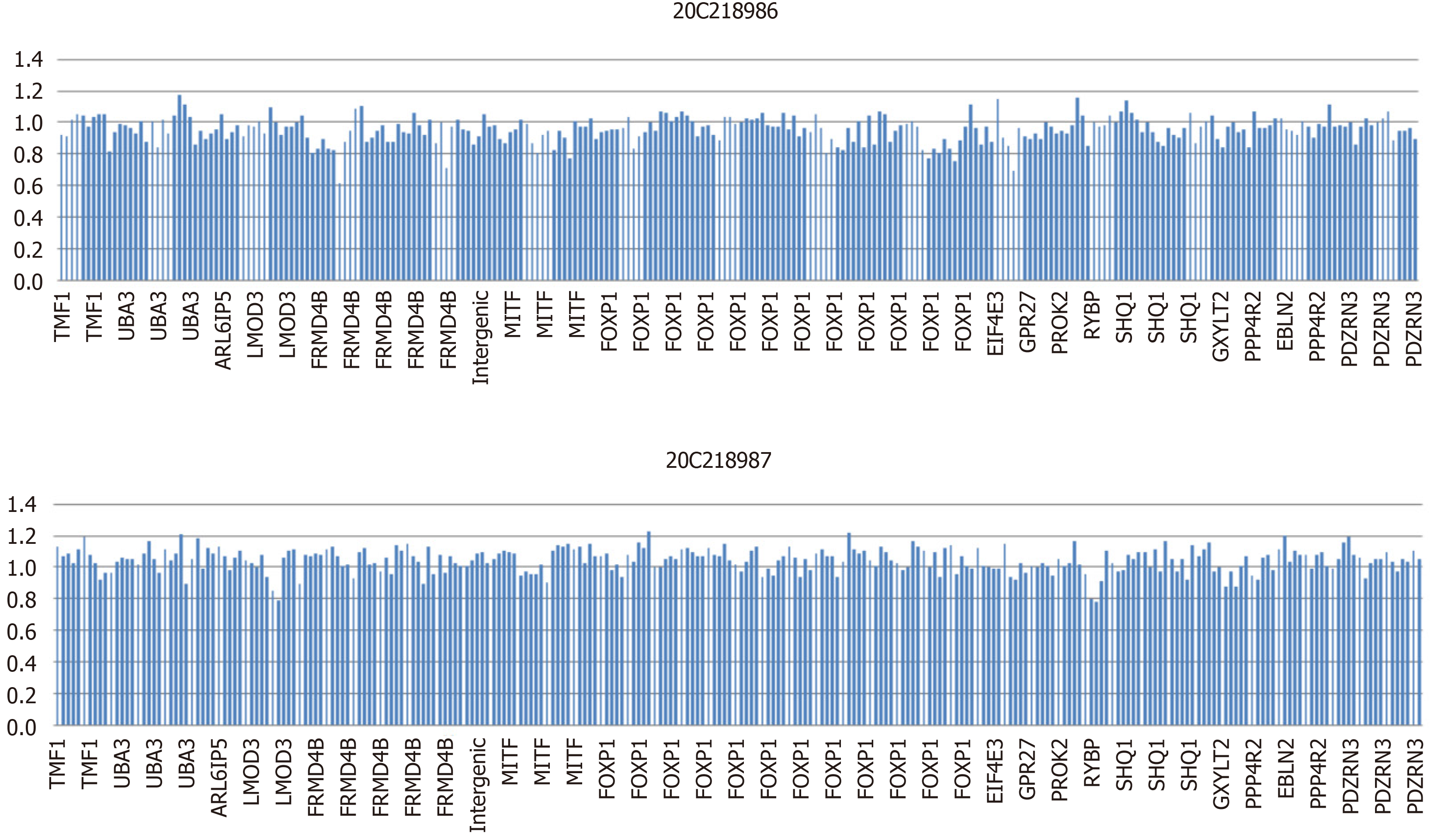
WES revealed a heterozygous deletion mutation in FOXP1 of the child, and this mutation was located at exons 6–21 and indicated a heterozygous deletion (Figure 1). This mutation was a zero effect (exon deletion) that may have resulted in loss of function of the gene. Moreover, the frequency of the mutation was negative relative to normal. Hence, it was a low-frequency mutation. However, as revealed by the pedigree, no mutation at the locus of the child’s father or mother was found (Figure 2). Thus, this mutation was spontaneous. According to the ACMG Guidelines, it was a pathogenic variant. No obvious genetic abnormalities were found in the CNV results.
The final diagnosis of the presented case is autism with dysphasia accompanied by mental retardation due to the heterozygous deletions of exon 6-21 of FOXP1 gene.
The patient, since his diagnosis, has been undergoing related speech and motor rehabilitation training in the hospital.
After treatment, the patient remained in Class I of S-S Language Delay Assessment on March 5, 2019: Language delay was still observed, and his developmental level was about 2.0–2.5-years-old. According to Gesell Developmental Schedules, his adaptive development quotient increased to 49.46 on March 6, 2019. His adaptability and personal-social skills were moderately lagging behind, and his other skills remained slightly lagging behind.
FOXP1 mutations may cause mental retardation accompanied by dysphasia in the absence or the presence of autism. FOXP1 mutations are manifested by dysphasia, motor retardation, mental retardation, stereotyped behaviors, hyperactivity, facial changes, heterotropia, nystagmus and obesity, with clinical symptoms progressing slowly but not affecting life expectancy. The child was diagnosed with autism with dysphasia accompanied by mental retardation because of FOXP1 mutations and the corresponding clinical manifestations with a better prognosis.
The FOX genome is a large and highly conservative family of transcription factors. FOXP, a subfamily of the FOX family, has four members, namely, FOXP1–FOXP4, which not only maintain a high degree of conservatism in structure but also participate in different developmental processes, such as neural development related to language and cognition, the formation and development of organs, such as the heart and lungs, and the development of the immune system[7-10].
As a member of the FOXP family, FOXP1 is widely and abundantly expressed in normal tissues early in the embryo. Banham et al[2,4] obtained a full-length cDNA encoding FOXP1, which is characterized by a DNA domain with a length of 100 amino acids and an evolutionarily conserved DNA domain called the forkhead or the forkhead/winged helix domain. Members of the FOXP subfamily have several atypical features in the FOX protein: The forkhead domain is located near the carboxyl terminal region, contains leucine zipper motifs that promote the homodimerization and heterodimerization of FOXP, and has a highly selective tissue- and cell type-specific activity.
In this case, the FOXP1 gene of the child had a heterozygous deletion variation, and the mutation site was heterozygous deletion in exons 6-21. Since the detection method was second generation WES, the regional deletion fracture could only be determined according to the region of exon captured by the gene. Therefore, we could only ascertain that the approximate location of the gene deletion in children was about 345 kb, the maximum deleted region is chr3: 71003864-71349076.
A total of 15 cases with FOXP1 mutations have been reported to date[11-20]. Eight cases had partial gene deletions, six had abnormal amino acid synthesis caused by base mutations, and one had abnormal amino acid synthesis and expression with a base (T) added (Table 1). Noticeable facial changes were observed in 10 of the reported cases and conduct disorders in 7 cases. No study has reported that FOXP1 mutations affect the lifespan of the patients; however, according to previous reports, this gene may have a tendency.
| Time of Publication | Age of the patient | Mutations in the positioning | Nucleic acid change | Amino acid change | Form of mutation | Language barrier | Motor retardation | Time to sit alone | Time to walk alone | Change in perception | Time to speak word | Face change | Stereotyped behavior |
| 2009 | 23 mo | Chr3:71164161-71958845 | + | + | 15 mo | 18 mo | + | 23 mo | + | NA | |||
| 2010 | 3 yr and 10 mo | Chr3:70549923-71618670 | Missing | + | + | NA | 16 mo | NA | 41 mo | + | NA | ||
| 2010 | 7 yr | Chr3:70807767-71305965 | Missing | + | + | NA | 24 mo | + | 42 mo | NA | NA | ||
| 5 yr and 6 mo | Chr3:71021785 | c.1573C>T | p.R525 | Variation | + | + | 12 mo | 36 mo | + | 42 mo | + | NA | |
| 6 yr | Chr3:tel:70341246-70341247 and cen:71388173-71388174 | Missing | + | + | 8 mo | 24 mo | + | 42 mo | + | NA | |||
| 2010 | 15 yr and 11 mo | Chr3:71114875-71504640/exons4-14 | Missing | + | + | NA | 18-20 mo | + | 3 yr | NA | + | ||
| 9 yr and 11 mo | 3p14.1 | c.1573C>T | p.R525X | Variation | + | + | NA | 18-20 mo | + | 6 yr | NA | + | |
| 2011 | NA | Chr3:71132860 | +T | p.Ala339SerfsX4 | Insert | + | + | NA | NA | + | NA | NA | + |
| 2012 | NA | Chr3:62133183-71072125 | Missing | + | + | NA | NA | + | NA | NA | NA | ||
| 2013 | 7 yr | Chr3:71041636-71229421 | Missing | + | + | 1 yr | 25 mo | + | 52 mo | + | NA | ||
| 2015 | 14 yr | Chr3:71027059-71027060 | c.1267-1268 | Missing | + | + | + | 13 mo | + | 20 mo | + | + | |
| 2014 | 2 yr | Chr3:71021758 | c.1600T>C | p.W534R | Variation | NA | NA | NA | NA | NA | NA | + | NA |
| 2016 | 11 yr | Chr3:71026829 | c.1393A>G | p.R465G | Variation | + | + | NA | 21 mo | + | NA | + | + |
| 7 yr | Chr3:71021818 | c.1540C>T | p.R514C | Variation | + | + | NA | 17 mo | + | NA | + | + | |
| 15 yr | Chr3:71027010 | c.1317C>G | p.Y439 | Variation | + | + | NA | 21 mo | + | NA | + | + | |
| This case | 5 yr | 3p14.1,exons6-21 | Missing | + | + | 9 mo | 2 yr | + | NA | + | + |
The closest patient to this case was a girl with a 390-kb deletion of exons 4-14 of her FOXP1 gene. As in this case, no abnormality was found in either parent[14]. The patient was not able to say words until she was 3-years-old, and since then she has been able to respond to language, but this response is mostly repeated language content, rather than language to answer questions. Despite the behavioral characteristics of autism, the patient had stereotyped behavior, compulsive behavior, hyperactivity, and was not diagnosed with autism.
Previous reports have shown that FOXP1 and FOXP2 (OMIM :605317) are closely related[13,19]. Both of them are homeostructures sharing similar amino terminal domains, and active in the foregut and brain in human development. The lack of FOXP1 and FOXP2 can be seen in the delay of language development (such as vocal retardation, dysarthria, grammatical monotony, etc.)[11], but FOXP2-related cases tend to describe the facial muscle fine motor dysfunction (such as lip, tongue muscle, etc.), whose variants are often accompanied by language expression and receptivity abnormalities, while FOXP1 tend to affect the nerves of children and lead to language retardation.
Analysis of the condition of the present case and those previously reported suggested that a certain correlation may exist between the FOXP1 mutation site and clinical phenotypes. Fifteen cases had dysgnosia, bradykinesia, and cognitive modifiability that affected motor development. However, compared with the abnormal expression caused by base mutation, patients with gene interruption are more likely to show more severe language and motor retardation, accompanied by limb deformities and organic lesions.
In children with facial changes, they often have the same type of performance: Giant malformation, wide eye distance, excessive strabismus, and wide nose tip[13,19]. In this case, the ear position of the child was slightly lower and there was a typical facial change. In some of the patients who had provided the relevant examination, some of the results showed abnormal structural development such as periventricular white matter, but there was no nervous system examination and EEG was abnormal. In this case, there was hydrocephalus during pregnancy. Postnatal examination showed that the right ventricle was dilated, and vacuolar sella and EEG were not abnormal. This may be related to the abnormal expression of FOXP1 gene deletion.
In addition to the motor retardation, some patients reported decreased muscle tension and referred to gait abnormalities in phenotypic description[20]. In this case, the children also had abnormal gait (such as walking instability, easy to fall), and achieved more obvious improvement after rehabilitation, which suggests that our timely and early intervention can improve the living standards of patients.
According to previous reports, children with clinical manifestations, such as dyskinesia, developmental retardation, dysphasia, cognitive modifiability and facial changes (low-set ears), meet the diagnostic criteria for phenotypic changes of mental retardation accompanied by dysphasia in the absence or the presence of autism. Notably, the illness episodes of Asian populations have never been defined or described in previously reported cases. Hence, the present case may provide new insights into the illness episodes and manifestations of a different race. Our clinical assessment of patients helps to describe the core phenotype of FOXP1-related disorders, which may contribute to disease identification.
In this study, WES was conducted to resolve three aspects. First, the etiology was established to clarify the genetic causes of the disease; this information is useful in genetic counseling. Second, genotype analysis is helpful to guide clinical treatment and prognosis evaluation, and modern rehabilitation training, such as educational intervention, behavior modification, speech therapy and sensory integration training, are conducive to early intervention and improvement in the quality of life of patients. Finally, the discovery of a new FOXP1 mutation with heterozygous deletions at exons 6–21 enriches our understanding of the FOXP1 mutation spectrum. The results of the present study suggest that investigation of intelligence development cannot be neglected for children with clinical manifestations of dysphasia and motor retardation. In such a case, the possibility of FOXP1 mutation should be raised.
We would like to thank the child and his family members for agreeing to participate in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iourov I S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Wang B, Lin D, Li C, Tucker P. Multiple domains define the expression and regulatory properties of Foxp1 forkhead transcriptional repressors. J Biol Chem. 2003;278:24259-24268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Banham AH, Beasley N, Campo E, Fernandez PL, Fidler C, Gatter K, Jones M, Mason DY, Prime JE, Trougouboff P, Wood K, Cordell JL. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820-8829. [PubMed] [Cited in This Article: ] |
| 3. | Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Banham AH, Connors JM, Brown PJ, Cordell JL, Ott G, Sreenivasan G, Farinha P, Horsman DE, Gascoyne RD. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11:1065-1072. [PubMed] [Cited in This Article: ] |
| 5. | Hu H, Wang B, Borde M, Nardone J, Maika S, Allred L, Tucker PW, Rao A. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13374] [Cited by in F6Publishing: 17951] [Article Influence: 1994.6] [Reference Citation Analysis (0)] |
| 7. | Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1827] [Cited by in F6Publishing: 1851] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 8. | Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Pääbo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 922] [Cited by in F6Publishing: 696] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 9. | Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, Tucker PW. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477-4487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Pariani MJ, Spencer A, Graham JM Jr, Rimoin DL. A 785kb deletion of 3p14.1p13, including the FOXP1 gene, associated with speech delay, contractures, hypertonia and blepharophimosis. Eur J Med Genet. 2009;52:123-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Carr CW, Moreno-De-Luca D, Parker C, Zimmerman HH, Ledbetter N, Martin CL, Dobyns WB, Abdul-Rahman OA. Chiari I malformation, delayed gross motor skills, severe speech delay, and epileptiform discharges in a child with FOXP1 haploinsufficiency. Eur J Hum Genet. 2010;18:1216-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Horn D, Kapeller J, Rivera-Brugués N, Moog U, Lorenz-Depiereux B, Eck S, Hempel M, Wagenstaller J, Gawthrope A, Monaco AP, Bonin M, Riess O, Wohlleber E, Illig T, Bezzina CR, Franke A, Spranger S, Villavicencio-Lorini P, Seifert W, Rosenfeld J, Klopocki E, Rappold GA, Strom TM. Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum Mutat. 2010;31:E1851-E1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, Langlois M, Foomani G, Dobrzeniecka S, Krebs MO, Joober R, Lafrenière RG, Lacaille JC, Mottron L, Drapeau P, Beauchamp MH, Phillips MS, Fombonne E, Rouleau GA, Michaud JL. De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet. 2010;87:671-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, Mackenzie AP, Ng SB, Baker C, Rieder MJ, Nickerson DA, Bernier R, Fisher SE, Shendure J, Eichler EE. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 870] [Cited by in F6Publishing: 827] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 16. | Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, Ernst C, Hanscom C, Rossin E, Lindgren AM, Pereira S, Ruderfer D, Kirby A, Ripke S, Harris DJ, Lee JH, Ha K, Kim HG, Solomon BD, Gropman AL, Lucente D, Sims K, Ohsumi TK, Borowsky ML, Loranger S, Quade B, Lage K, Miles J, Wu BL, Shen Y, Neale B, Shaffer LG, Daly MJ, Morton CC, Gusella JF. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell. 2012;149:525-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 425] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 17. | Le Fevre AK, Taylor S, Malek NH, Horn D, Carr CW, Abdul-Rahman OA, O'Donnell S, Burgess T, Shaw M, Gecz J, Bain N, Fagan K, Hunter MF. FOXP1 mutations cause intellectual disability and a recognizable phenotype. Am J Med Genet A. 2013;161A:3166-3175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Srivastava S, Cohen JS, Vernon H, Barañano K, McClellan R, Jamal L, Naidu S, Fatemi A. Clinical whole exome sequencing in child neurology practice. Ann Neurol. 2014;76:473-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Lozano R, Vino A, Lozano C, Fisher SE, Deriziotis P. A de novo FOXP1 variant in a patient with autism, intellectual disability and severe speech and language impairment. Eur J Hum Genet. 2015;23:1702-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Sollis E, Graham SA, Vino A, Froehlich H, Vreeburg M, Dimitropoulou D, Gilissen C, Pfundt R, Rappold GA, Brunner HG, Deriziotis P, Fisher SE. Identification and functional characterization of de novo FOXP1 variants provides novel insights into the etiology of neurodevelopmental disorder. Hum Mol Genet. 2016;25:546-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |