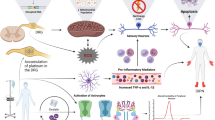
Abstract
Chemotherapy-induced peripheral neurotoxicity (CIPN) is a major dose-limiting side effect of many anti-cancer agents, including taxanes, platinums, vinca alkaloids, proteasome inhibitors, immunomodulatory drugs, and antibody–drug conjugates. The resultant symptoms often persist post treatment completion and continue to impact on long-term function and quality of life for cancer survivors. At present, dose reduction remains the only strategy to prevent severe neuropathy, often leading clinicians to the difficult decision of balancing maximal treatment exposure and minimal long-lasting side effects. This review examines the clinical presentations of CIPN with each class of neurotoxic treatment, describing signs, symptoms, and long-term outcomes. We provide an update on the proposed mechanisms of nerve damage and review current data on clinical and genetic risk factors contributing to CIPN development. We also examine recent areas of research in the treatment and prevention of CIPN, with specific focus on current clinical trials and consensus recommendations for CIPN management.
Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M et al (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 63(6):419–437. https://doi.org/10.3322/caac.21204
Cavaletti G, Marmiroli P (2018) Pharmacotherapy options for managing chemotherapy-induced peripheral neurotoxicity. Expert Opin Pharmacother 19(2):113–121. https://doi.org/10.1080/14656566.2017.1415326
Hennenfent KL, Govindan R (2006) Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 17(5):735–49. https://doi.org/10.1093/annonc/mdj100
Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D et al (2011) Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 125(3):767–774. https://doi.org/10.1007/s10549-010-1278-0
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671. https://doi.org/10.1056/NEJMoa0707056
Cortot AB, Audigier-Valette C, Molinier O, Le Moulec S, Barlesi F, Zalcman G, Dumont P et al (2020) Weekly paclitaxel plus bevacizumab versus docetaxel as second- or third-line treatment in advanced non-squamous non-small-cell lung cancer: results of the IFCT-1103 ULTIMATE study. Eur J Cancer 131:27–36. https://doi.org/10.1016/j.ejca.2020.02.022
Swain SM, Arezzo JC (2008) Neuropathy associated with microtubule inhibitors: diagnosis, incidence, and management. Clin Adv Hematol Oncol 6(6):455–467
Timmins HC, Li T, Trinh T, Kiernan MC, Harrison M, Boyle F, Friedlander M et al (2021) Weekly paclitaxel-induced neurotoxicity in breast cancer: outcomes and dose response. Oncologist 26(5):366–374. https://doi.org/10.1002/onco.13697
Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ (1995) Paclitaxel-induced neuropathy. Ann Oncol 6(5):489–494. https://doi.org/10.1093/oxfordjournals.annonc.a059220
Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 66(3):218–228. https://doi.org/10.1016/j.critrevonc.2008.01.008
Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A et al (2011) Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol 29(11):1472–1478. https://doi.org/10.1200/jco.2010.33.0308
Velasco R, Bruna J (2015) Taxane-induced peripheral neurotoxicity. Toxics 3(2):152–169. https://doi.org/10.3390/toxics3020152
Pachman DR, Qin R, Seisler D, Smith EM, Kaggal S, Novotny P, Ruddy KJ et al (2016) Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 24(12):5059–5068. https://doi.org/10.1007/s00520-016-3373-1
Hilkens PH, Verweij J, Vecht CJ, Stoter G, van den Bent MJ (1997) Clinical characteristics of severe peripheral neuropathy induced by docetaxel (Taxotere). Ann Oncol 8(2):187–190. https://doi.org/10.1023/a:1008245400251
Argyriou AA, Briani C, Cavaletti G, Bruna J, Alberti P, Velasco R, Lonardi S et al (2013) Advanced age and liability to oxaliplatin-induced peripheral neuropathy: post hoc analysis of a prospective study 20(5):788–794. https://doi.org/10.1111/ene.12061
Staff NP, Cavaletti G, Islam B, Lustberg M, Psimaras D, Tamburin S (2019) Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 24(Suppl 2):S26-s39. https://doi.org/10.1111/jns.12335
Quasthoff S, Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249(1):9–17. https://doi.org/10.1007/pl00007853
Pachman DR, Qin R, Seisler DK, Smith EM, Beutler AS, Ta LE, Lafky JM et al (2015) Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 33(30):3416–3422. https://doi.org/10.1200/jco.2014.58.8533
Beijers AJ, Mols F, Vreugdenhil G (2014) A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 22(7):1999–2007. https://doi.org/10.1007/s00520-014-2242-z
Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D (2012) Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer 20(11):2959–2967. https://doi.org/10.1007/s00520-012-1428-5
Albany C, Dockter T, Wolfe E, Le-Rademacher J, Wagner-Johnston N, Einhorn L, Lafky JM et al (2021) Cisplatin-associated neuropathy characteristics compared with those associated with other neurotoxic chemotherapy agents (Alliance A151724). Support Care Cancer 29(2):833–840. https://doi.org/10.1007/s00520-020-05543-5
Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC (2011) Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility. Oncologist 16(5):708–716. https://doi.org/10.1634/theoncologist.2010-0248
Briani C, Argyriou AA, Izquierdo C, Velasco R, Campagnolo M, Alberti P, Frigeni B et al (2014) Long-term course of oxaliplatin-induced polyneuropathy: a prospective 2-year follow-up study. J Peripher Nerv Syst 19(4):299–306. https://doi.org/10.1111/jns.12097
Ness KK, Jones KE, Smith WA, Spunt SL, Wilson CL, Armstrong GT, Srivastava DK et al (2013) Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 94(8):1451–7. https://doi.org/10.1016/j.apmr.2013.03.009
Selvy M, Pereira B, Kerckhove N, Gonneau C, Feydel G, Pétorin C, Vimal-Baguet A et al (2020) Long-term prevalence of sensory chemotherapy-induced peripheral neuropathy for 5 years after adjuvant FOLFOX chemotherapy to treat colorectal cancer: a multicenter cross-sectional study. J Clin Med. 9(8):2400. https://doi.org/10.3390/jcm9082400
Yoshino T, Yamanaka T, Oki E, Kotaka M, Manaka D, Eto T, Hasegawa J et al (2019) Efficacy and long-term peripheral sensory neuropathy of 3 vs 6 months of oxaliplatin-based adjuvant chemotherapy for colon cancer: the ACHIEVE phase 3 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.2572
Noble RL (1990) The discovery of the vinca alkaloids–chemotherapeutic agents against cancer. Biochem Cell Biol 68(12):1344–1351
Islam B, Lustberg M, Staff NP, Kolb N, Alberti P, Argyriou AA (2019) Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst 24(Suppl 2):S63-s73. https://doi.org/10.1111/jns.12334
Haim N, Epelbaum R, Ben-Shahar M, Yarnitsky D, Simri W, Robinson E (1994) Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer 73(10):2515–2519. https://doi.org/10.1002/1097-0142(19940515)73:10%3c2515::aid-cncr2820731011%3e3.0.co;2-g
Li T, Timmins HC, Lazarus HM, Park SB (2020) Peripheral neuropathy in hematologic malignancies – past, present and future. Blood Rev 43:100653. https://doi.org/10.1016/j.blre.2020.100653
Verstappen CC, Koeppen S, Heimans JJ, Huijgens PC, Scheulen ME, Strumberg D, Kiburg B et al (2005) Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology 64(6):1076–1077. https://doi.org/10.1212/01.Wnl.0000154642.45474.28
Lavoie Smith EM, Li L, Chiang C, Thomas K, Hutchinson RJ, Wells EM, Ho RH et al (2015) Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst 20(1):37–46. https://doi.org/10.1111/jns.12114
Gilchrist LS, Tanner LR, Ness KK (2017) Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer 64(1):180–187. https://doi.org/10.1002/pbc.26204
Tay CG, Lee V, Ong LC, Goh KJ, Ariffin H, Fong CY (2017) Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 64(8):e26471. https://doi.org/10.1002/pbc.26471
Kandula T, Farrar MA, Cohn RJ, Mizrahi D, Carey K, Johnston K, Kiernan MC et al (2018) Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.0963
Li T, Timmins HC, King T, Kiernan MC, Goldstein D, Park SB (2020) Characteristics and risk factors of bortezomib induced peripheral neuropathy: a systematic review of phase III trials. Hematol Oncol 38(3):229–243. https://doi.org/10.1002/hon.2706
Hu B, Zhou Q, Wu T, Zhuang L, Yi L, Cao J, Yang X et al (2017) Efficacy and safety of subcutaneous versus intravenous bortezomib in multiple myeloma: a meta-analysis. Int J Clin Pharmacol Ther 55(4):329–338. https://doi.org/10.5414/cp202714
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I et al (2016) Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375(8):754–766. https://doi.org/10.1056/NEJMoa1606038
Richardson PG, Laubach JP, Schlossman RL, Mitsiades C, Anderson K (2010) Complications of multiple myeloma therapy, part 1: risk reduction and management of peripheral neuropathy and asthenia. J Natl Compr Canc Netw 8(Suppl 1):S4-s12. https://doi.org/10.6004/jnccn.2010.0115
Stratogianni A, Tosch M, Schlemmer H, Weis J, Katona I, Isenmann S, Haensch CA (2012) Bortezomib-induced severe autonomic neuropathy. Clin Auton Res 22(4):199–202. https://doi.org/10.1007/s10286-012-0164-8
Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D et al (2009) Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol 144(6):895–903. https://doi.org/10.1111/j.1365-2141.2008.07573.x
Tacchetti P, Terragna C, Galli M, Zamagni E, Petrucci MT, Pezzi A, Montefusco V et al (2014) Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: clinical and molecular analyses of a phase 3 study. Am J Hematol 89(12):1085–1091. https://doi.org/10.1002/ajh.23835
Delforge M, Bladé J, Dimopoulos MA, Facon T, Kropff M, Ludwig H, Palumbo A et al (2010) Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol 11(11):1086–1095. https://doi.org/10.1016/s1470-2045(10)70068-1
van de Donk NW, van der Holt B, Minnema MC, Vellenga E, Croockewit S, Kersten MJ, von dem Borne PA et al (2018) Thalidomide before and after autologous stem cell transplantation in recently diagnosed multiple myeloma (HOVON-50): long-term results from the phase 3, randomised controlled trial. Lancet Haematol 5(10):e479–e492. https://doi.org/10.1016/s2352-3026(18)30149-2
Marriott JB, Muller G, Stirling D, Dalgleish AG (2001) Immunotherapeutic and antitumour potential of thalidomide analogues. Expert Opin Biol Ther 1(4):675–682. https://doi.org/10.1517/14712598.1.4.675
Zweegman S, van der Holt B, Mellqvist UH, Salomo M, Bos GM, Levin MD, Visser-Wisselaar H et al (2016) Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood 127(9):1109–1116. https://doi.org/10.1182/blood-2015-11-679415
Miguel JS, Weisel K, Moreau P, Lacy M, Song K, Delforge M, Karlin L et al (2013) Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 14(11):1055–1066. https://doi.org/10.1016/s1470-2045(13)70380-2
Mileshkin L, Stark R, Day B, Seymour JF, Zeldis JB, Prince HM (2006) Development of neuropathy in patients with myeloma treated with thalidomide: patterns of occurrence and the role of electrophysiologic monitoring. J Clin Oncol 24(27):4507–4514. https://doi.org/10.1200/jco.2006.05.6689
Fahdi IE, Gaddam V, Saucedo JF, Kishan CV, Vyas K, Deneke MG, Razek H et al (2004) Bradycardia during therapy for multiple myeloma with thalidomide. Am J Cardiol 93(8):1052–1055. https://doi.org/10.1016/j.amjcard.2003.12.061
Stork M, Sandecká V, Boichuk I, Adam Z, Krejci M, Brozova L, Sevcikova S et al (2019) Bortezomib and thalidomide treatment results in newly diagnosed transplant-ineligible multiple myeloma patients are comparable in long-term follow-up. Klin Onkol. 32(6):445–52. https://doi.org/10.14735/amko2019445 ((Výsledky léčby bortezomibem a thalidomidem u nově diagnostikovaných netransplantovaných pacientů s mnohočetným myelomem jsou srovnatelné.))
Dalla Torre C, Zambello R, Cacciavillani M, Campagnolo M, Berno T, Salvalaggio A, De March E et al (2016) Lenalidomide long-term neurotoxicity: clinical and neurophysiologic prospective study. Neurology 87(11):1161–1166. https://doi.org/10.1212/wnl.0000000000003093
Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A et al (2018) Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med 378(4):331–344. https://doi.org/10.1056/NEJMoa1708984
Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL et al (2019) Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393(10168):229–240. https://doi.org/10.1016/s0140-6736(18)32984-2
Fanale MA, Whiting NC, Neylon E (2015) Treatment strategies to optimize outcomes with brentuximab vedotin: management of common and rare toxicities. J Target Ther Cancer 4(2):36–45
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI et al (2015) Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 385(9980):1853–1862. https://doi.org/10.1016/s0140-6736(15)60165-9
Mariotto S, Ferrari S, Sorio M, Benedetti F, Tridente G, Cavallaro T, Gajofatto A et al (2015) Brentuximab vedotin: axonal microtubule’s Apollyon. Blood Cancer J 5(8):e343. https://doi.org/10.1038/bcj.2015.72
Corbin ZA, Nguyen-Lin A, Li S, Rahbar Z, Tavallaee M, Vogel H, Salva KA et al (2017) Characterization of the peripheral neuropathy associated with brentuximab vedotin treatment of mycosis Fungoides and Sézary syndrome. J Neurooncol 132(3):439–446. https://doi.org/10.1007/s11060-017-2389-9
Tolaney SM, Tayob N, Dang C, Yardley DA, Isakoff SJ, Valero V, Faggen M et al (2021) Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J Clin Oncol 39(21):2375–2385. https://doi.org/10.1200/JCO.20.03398
Tamburin S, Park SB, Alberti P, Demichelis C, Schenone A, Argyriou AA (2019) Taxane and epothilone-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst 24(Suppl 2):S40-s51. https://doi.org/10.1111/jns.12336
Vahdat LT, Thomas ES, Roché HH, Hortobagyi GN, Sparano JA, Yelle L, Fornier MN et al (2012) Ixabepilone-associated peripheral neuropathy: data from across the phase II and III clinical trials. Support Care Cancer 20(11):2661–2668. https://doi.org/10.1007/s00520-012-1384-0
Fukuda Y, Li Y, Segal RA (2017) A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci 11:481. https://doi.org/10.3389/fnins.2017.00481
Figley MD, Gu W, Nanson JD, Shi Y, Sasaki Y, Cunnea K, Malde AK et al (2021) SARM1 is a metabolic sensor activated by an increased NMN/NAD(+) ratio to trigger axon degeneration. Neuron 109(7):1118–36.e11. https://doi.org/10.1016/j.neuron.2021.02.009
Turkiew E, Falconer D, Reed N, Höke A (2017) Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J Peripher Nerv Syst 22(3):162–171. https://doi.org/10.1111/jns.12219
Gould SA, White M, Wilbrey AL, Pór E, Coleman MP, Adalbert R (2021) Protection against oxaliplatin-induced mechanical and thermal hypersensitivity in Sarm1(-/-) mice. Exp Neurol 338:113607. https://doi.org/10.1016/j.expneurol.2021.113607
Geisler S (2021) Vincristine- and bortezomib-induced neuropathies - from bedside to bench and back. Exp Neurol 336:113519. https://doi.org/10.1016/j.expneurol.2020.113519
Gill JS, Windebank AJ (1998) Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest 101(12):2842–2850. https://doi.org/10.1172/jci1130
Ta LE, Espeset L, Podratz J, Windebank AJ (2006) Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology 27(6):992–1002. https://doi.org/10.1016/j.neuro.2006.04.010
Stage TB, Hu S, Sparreboom A, Kroetz DL (2021) Role for drug transporters in chemotherapy-induced peripheral neuropathy. Clin Transl Sci 14(2):460–467. https://doi.org/10.1111/cts.12915
Canta A, Pozzi E, Carozzi VA (2015) Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 3(2):198–223. https://doi.org/10.3390/toxics3020198
Bobylev I, Joshi AR, Barham M, Neiss WF, Lehmann HC (2018) Depletion of mitofusin-2 causes mitochondrial damage in cisplatin-induced neuropathy. Mol Neurobiol 55(2):1227–1235. https://doi.org/10.1007/s12035-016-0364-7
Flatters SJL, Bennett GJ (2006) Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122(3):245–257. https://doi.org/10.1016/j.pain.2006.01.037
Duggett NA, Griffiths LA, McKenna OE, de Santis V, Yongsanguanchai N, Mokori EB, Flatters SJ (2016) Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 333:13–26. https://doi.org/10.1016/j.neuroscience.2016.06.050
Cirrincione AM, Pellegrini AD, Dominy JR, Benjamin ME, Utkina-Sosunova I, Lotti F, Jergova S et al (2020) Paclitaxel-induced peripheral neuropathy is caused by epidermal ROS and mitochondrial damage through conserved MMP-13 activation. Sci Rep 10(1):3970. https://doi.org/10.1038/s41598-020-60990-8
Bobylev I, Joshi AR, Barham M, Ritter C, Neiss WF, Höke A, Lehmann HC (2015) Paclitaxel inhibits mRNA transport in axons. Neurobiol Dis 82:321–331. https://doi.org/10.1016/j.nbd.2015.07.006
Duggett NA, Griffiths LA, Flatters SJL (2017) Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain 158(8):1499–1508. https://doi.org/10.1097/j.pain.0000000000000939
Argyriou AA, Bruna J, Park SB, Cavaletti G (2020) Emerging pharmacological strategies for the management of chemotherapy-induced peripheral neurotoxicity (CIPN), based on novel CIPN mechanisms. Expert Rev Neurother. 20(10):1005–16. https://doi.org/10.1080/14737175.2020.1796639
LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA (2013) Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology 37:231–239. https://doi.org/10.1016/j.neuro.2013.05.008
Topp KS, Tanner KD, Levine JD (2000) Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol 424(4):563–576
Alé A, Bruna J, Herrando M, Navarro X, Udina E (2015) Toxic effects of bortezomib on primary sensory neurons and Schwann cells of adult mice. Neurotox Res 27(4):430–440. https://doi.org/10.1007/s12640-014-9514-8
Mariotto S, Tecchio C, Sorio M, Bertolasi L, Turatti M, Tozzi MC, Benedetti F et al (2019) Clinical and neurophysiological serial assessments of brentuximab vedotin-associated peripheral neuropathy. Leuk Lymphoma 60(11):2806–2809. https://doi.org/10.1080/10428194.2019.1605068
Lees JG, Makker PG, Tonkin RS, Abdulla M, Park SB, Goldstein D, Moalem-Taylor G (2017) Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer 73:22–29. https://doi.org/10.1016/j.ejca.2016.12.006
Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C (2020) Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol 11:626687. https://doi.org/10.3389/fimmu.2020.626687
Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM (2016) Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain 17(7):775–786. https://doi.org/10.1016/j.jpain.2016.02.011
Singh SK, Spiegel S (2020) Sphingosine-1-phosphate signaling: a novel target for simultaneous adjuvant treatment of triple negative breast cancer and chemotherapy-induced neuropathic pain. Adv Biol Regul 75:100670. https://doi.org/10.1016/j.jbior.2019.100670
Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS Jr (2001) Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 276(25):22382–22387. https://doi.org/10.1074/jbc.M100938200
Robertson J, Raizer J, Hodges JS, Gradishar W, Allen JA (2018) Risk factors for the development of paclitaxel-induced neuropathy in breast cancer patients. J Peripher Nerv Syst 23(2):129–133. https://doi.org/10.1111/jns.12271
Molassiotis A, Cheng HL, Lopez V, Au JSK, Chan A, Bandla A, Leung KT et al (2019) Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 19(1):132. https://doi.org/10.1186/s12885-019-5302-4
Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, Minasian LM et al (2016) Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in southwest oncology group clinical trials. J Clin Oncol 34(25):3014–3022. https://doi.org/10.1200/jco.2015.66.2346
Mizrahi D, Park SB, Li T, Timmins HC, Trinh T, Au K, Battaglini E et al (2021) Hemoglobin, body mass index, and age as risk factors for paclitaxel- and oxaliplatin-induced peripheral neuropathy. JAMA Network Open. 4(2):e2036695-e. https://doi.org/10.1001/jamanetworkopen.2020.36695
Molassiotis A, Cheng HL, Leung KT, Li YC, Wong KH, Au JSK, Sundar R et al (2019) Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain and behavior 9(6):e01312. https://doi.org/10.1002/brb3.1312
Timmins HC, Mizrahi D, Li T, Kiernan MC, Goldstein D, Park SB (2021) Metabolic and lifestyle risk factors for chemotherapy-induced peripheral neuropathy in taxane and platinum-treated patients: a systematic review. J Cancer Surviv. https://doi.org/10.1007/s11764-021-00988-x
Barginear M, Dueck AC, Allred JB, Bunnell C, Cohen HJ, Freedman RA, Hurria A et al (2019) Age and the risk of paclitaxel-induced neuropathy in women with early-stage breast cancer (alliance A151411): results from 1,881 patients from cancer and leukemia group b (CALGB) 40101. Oncologist 24(5):617–623. https://doi.org/10.1634/theoncologist.2018-0298
Vincenzi B, Frezza AM, Schiavon G, Spoto C, Silvestris N, Addeo R, Catalano V et al (2013) Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer 21(5):1313–1319. https://doi.org/10.1007/s00520-012-1667-5
Gu J, Lu H, Chen C, Gu Z, Hu M, Liu L, Yu J et al (2021) Diabetes mellitus as a risk factor for chemotherapy-induced peripheral neuropathy: a meta-analysis. Support Care Cancer. https://doi.org/10.1007/s00520-021-06321-7
Battaglini E, Goldstein D, Grimison P, McCullough S, Mendoza-Jones P, Park SB (2021) Chemotherapy-induced peripheral neurotoxicity in cancer survivors: predictors of long-term patient outcomes. J Natl Compr Canc Netw 19(7):821–828. https://doi.org/10.6004/jnccn.2021.7026
Ibañez-Juliá MJ, Berzero G, Reyes-Botero G, Maisonobe T, Lenglet T, Slim M, Louis S et al (2018) Antineoplastic agents exacerbating Charcot Marie tooth disease: red flags to avoid permanent disability. Acta Oncol 57(3):403–411. https://doi.org/10.1080/0284186X.2017.1415462
Sánchez-Barroso L, Apellaniz-Ruiz M, Gutiérrez-Gutiérrez G, Santos M, Roldán-Romero JM, Curras M, Remacha L et al (2019) Concomitant medications and risk of chemotherapy-induced peripheral neuropathy. Oncologist 24(8):e784–e792. https://doi.org/10.1634/theoncologist.2018-0418
van Schie RM, Brüggemann RJ, Hoogerbrugge PM, te Loo DM (2011) Effect of azole antifungal therapy on vincristine toxicity in childhood acute lymphoblastic leukaemia. J Antimicrob Chemother 66(8):1853–1856. https://doi.org/10.1093/jac/dkr223
Zirpoli GR, McCann SE, Sucheston-Campbell LE, Hershman DL, Ciupak G, Davis W, Unger JM et al (2017) Supplement use and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221): the DELCaP study. J Natl Cancer Inst 109(12):djx098. https://doi.org/10.1093/jnci/djx098
Velasco R, Santos C, Soler G, Gil-Gil M, Pernas S, Galan M, Palmero R et al (2016) Serum micronutrients and prealbumin during development and recovery of chemotherapy-induced peripheral neuropathy. J Peripher Nerv Syst 21(3):134–141. https://doi.org/10.1111/jns.12177
Yildirim N, Cengiz M (2020) Predictive clinical factors of chronic peripheral neuropathy induced by oxaliplatin. Support Care Cancer. https://doi.org/10.1007/s00520-020-05319-x
Saito T, Okamura A, Inoue J, Makiura D, Doi H, Yakushijin K, Matsuoka H et al (2019) Anemia is a novel predictive factor for the onset of severe chemotherapy-induced peripheral neuropathy in lymphoma patients receiving rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy. Oncology research. 27(4):469–74. https://doi.org/10.3727/096504018x15267574931782
Argyriou AA, Bruna J, Genazzani AA, Cavaletti G (2017) Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat Rev Neurol 13(8):492–504. https://doi.org/10.1038/nrneurol.2017.88
Cliff J, Jorgensen AL, Lord R, Azam F, Cossar L, Carr DF, Pirmohamed M (2017) The molecular genetics of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Crit Rev Oncol Hematol 120:127–140. https://doi.org/10.1016/j.critrevonc.2017.09.009
Savas S, Kim DY, Ahmad MF, Shariff M, Ozcelik H (2004) Identifying functional genetic variants in DNA repair pathway using protein conservation analysis. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res Am Soc Prev Oncol 13(5):801–807
Zhang X, Jiang LP, Yin Y, Wang YD (2014) XRCC1 and XPD genetic polymorphisms and clinical outcomes of gastric cancer patients treated with oxaliplatin-based chemotherapy: a meta-analysis. Tumour Biol 35(6):5637–5645. https://doi.org/10.1007/s13277-014-1746-y
Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M et al (2001) XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104(1):107–117. https://doi.org/10.1016/s0092-8674(01)00195-7
Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, Vallier AL et al (2014) Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel. Clin Cancer Res 20(9):2466–2475. https://doi.org/10.1158/1078-0432.Ccr-13-3232
Wright GEB, Amstutz U, Drögemöller BI, Shih J, Rassekh SR, Hayden MR, Carleton BC et al (2019) Pharmacogenomics of vincristine-induced peripheral neuropathy implicates pharmacokinetic and inherited neuropathy genes. Clin Pharmacol Ther 105(2):402–410. https://doi.org/10.1002/cpt.1179
Chua KC, Xiong C, Ho C, Mushiroda T, Jiang C, Mulkey F, Lai D et al (2020) Genomewide meta-analysis validates a role for S1PR1 in microtubule targeting agent-induced sensory peripheral neuropathy. Clin Pharmacol Ther 108(3):625–634. https://doi.org/10.1002/cpt.1958
Peila E, D’Agata F, Caroppo P, Orsi L, Mortara P, Cauda S, Manfredi M et al (2016) Chemotherapy-induced neurotoxicity: evidence of a protective role of CC homozygosis in the interleukin-1β gene-511 C>T polymorphism. Neurotox Res 30(3):521–529. https://doi.org/10.1007/s12640-016-9637-1
Leandro-García LJ, Leskelä S, Jara C, Gréen H, Avall-Lundqvist E, Wheeler HE, Dolan ME et al (2012) Regulatory polymorphisms in β-tubulin IIa are associated with paclitaxel-induced peripheral neuropathy. Clin Cancer Res 18(16):4441–4448. https://doi.org/10.1158/1078-0432.Ccr-12-1221
Chan A, Hertz DL, Morales M, Adams EJ, Gordon S, Tan CJ, Staff NP et al (2019) Biological predictors of chemotherapy-induced peripheral neuropathy (CIPN): MASCC neurological complications working group overview. Support Care Cancer 27(10):3729–3737. https://doi.org/10.1007/s00520-019-04987-8
Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, Zheng JJ et al (2015) Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 313(8):815–823. https://doi.org/10.1001/jama.2015.0894
Apellániz-Ruiz M, Sánchez-Barroso L, Gutiérrez-Gutiérrez G, Sereno M, García-Donás J, Åvall-Lundqvist E, Gréen H et al (2015) Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel–letter. Clin Cancer Res 21(13):3092–3093. https://doi.org/10.1158/1078-0432.Ccr-14-1885
Leandro-García LJ, Inglada-Pérez L, Pita G, Hjerpe E, Leskelä S, Jara C, Mielgo X et al (2013) Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet 50(9):599–605. https://doi.org/10.1136/jmedgenet-2012-101466
Apellániz-Ruiz M, Tejero H, Inglada-Pérez L, Sánchez-Barroso L, Gutiérrez-Gutiérrez G, Calvo I, Castelo B et al (2017) Targeted sequencing reveals low-frequency variants in epha genes as markers of paclitaxel-induced peripheral neuropathy. Clin Cancer Res 23(5):1227–1235. https://doi.org/10.1158/1078-0432.Ccr-16-0694
Hertz DL, Owzar K, Lessans S, Wing C, Jiang C, Kelly WK, Patel J et al (2016) Pharmacogenetic discovery in CALGB (Alliance) 90401 and mechanistic validation of a VAC14 polymorphism that increases risk of docetaxel-induced neuropathy. Clin Cancer Res 22(19):4890–4900. https://doi.org/10.1158/1078-0432.Ccr-15-2823
Adjei AA, Lopez CL, Schaid DJ, Sloan JA, Le-Rademacher JG, Loprinzi CL, Norman AD et al (2021) Genetic predictors of chemotherapy-induced peripheral neuropathy from paclitaxel, carboplatin and oxaliplatin: NCCTG/alliance N08C1, N08CA and N08CB study. Cancers (Basel) 13(5):1084. https://doi.org/10.3390/cancers13051084
Magrangeas F, Kuiper R, Avet-Loiseau H, Gouraud W, Guérin-Charbonnel C, Ferrer L, Aussem A et al (2016) A genome-wide association study identifies a novel locus for bortezomib-induced peripheral neuropathy in european patients with multiple myeloma. Clin Cancer Res 22(17):4350–4355. https://doi.org/10.1158/1078-0432.Ccr-15-3163
Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, Kelley MR et al (2020) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clinical Oncology. 0(0):JCO.20.01399. https://doi.org/10.1200/jco.20.01399
Jordan B, Margulies A, Cardoso F, Cavaletti G, Haugnes HS, Jahn P, Le Rhun E et al (2020) Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol 31(10):1306–1319. https://doi.org/10.1016/j.annonc.2020.07.003
Hershman DL, Unger JM, Crew KD, Till C, Greenlee H, Minasian LM, Moinpour CM et al (2018) Two-year trends of taxane-induced neuropathy in women enrolled in a randomized trial of acetyl-l-carnitine (SWOG S0715). J Natl Cancer Inst 110(6):669–676. https://doi.org/10.1093/jnci/djx259
Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR et al (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 309(13):1359–1367. https://doi.org/10.1001/jama.2013.2813
Farshchian N, Alavi A, Heydarheydari S, Moradian N (2018) Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother Pharmacol 82(5):787–793. https://doi.org/10.1007/s00280-018-3664-y
Salehifar E, Janbabaei G, Hendouei N, Alipour A, Tabrizi N, Avan R (2020) Comparison of the efficacy and safety of pregabalin and duloxetine in taxane-induced sensory neuropathy: a randomized controlled trial. Clin Drug Investig 40(3):249–257. https://doi.org/10.1007/s40261-019-00882-6
Hirayama Y, Ishitani K, Sato Y, Iyama S, Takada K, Murase K, Kuroda H et al (2015) Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol 20(5):866–871. https://doi.org/10.1007/s10147-015-0810-y
Trinh T, Park SB, Murray J, Pickering H, Lin CS, Martin A, Friedlander M et al (2021) Neu-horizons: neuroprotection and therapeutic use of riluzole for the prevention of oxaliplatin-induced neuropathy-a randomised controlled trial. Support Care Cancer 29(2):1103–1110. https://doi.org/10.1007/s00520-020-05591-x
Timmins HC, Li T, Kiernan MC, Horvath LG, Goldstein D, Park SB (2020) Quantification of small fiber neuropathy in chemotherapy-treated patients. J Pain 21(1–2):44–58. https://doi.org/10.1016/j.jpain.2019.06.011
Guo X, Sun H, Dong J, Feng Y, Li H, Zhuang R, Wang P et al (2019) Does nab-paclitaxel have a higher incidence of peripheral neuropathy than solvent-based paclitaxel? Evidence from a systematic review and meta-analysis. Crit Rev Oncol Hematol 139:16–23. https://doi.org/10.1016/j.critrevonc.2019.04.021
Goldstein D, Von Hoff DD, Moore M, Greeno E, Tortora G, Ramanathan RK, Macarulla T et al (2016) Development of peripheral neuropathy and its association with survival during treatment with nab-paclitaxel plus gemcitabine for patients with metastatic adenocarcinoma of the pancreas: a subset analysis from a randomised phase III trial (MPACT). Eur J Cancer 52:85–91. https://doi.org/10.1016/j.ejca.2015.10.017
Funding
This study was supported by a Cancer Institute NSW Program Grant (14/TPG/1–05) and a National Health and Medical Research Council of Australia (NHMRC) Project Grant (#1080521). SBP is supported by a NHMRC Career Development Fellowship (#1148595). MCK receives funding from NHMRC Program Grant (#1132524), Partnership Project (#1153439) and is supported by a NHMRC Practitioner Fellowship (#1156093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, T., Mizrahi, D., Goldstein, D. et al. Chemotherapy and peripheral neuropathy. Neurol Sci 42, 4109–4121 (2021). https://doi.org/10.1007/s10072-021-05576-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05576-6