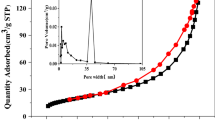
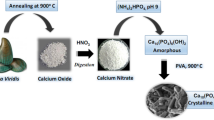
Abstract
Uranium is a naturally occurring trace element and radionuclide. Uranium is introduced in the environment during industrial activities and nuclear energy accidents involving nuclear power plants, nuclear weapons tests, ore mining, and manufacturing, which may lead to the contamination of groundwater and soil. Hydroxyapatite (HAP) is a natural mineral with a high affinity for uranium in water. Groundwater often contains high carbonate concentrations that may affect uranium removal due to the formation of uranyl carbonate complexes. In order to understand the process of uranium removal, uranium adsorptions on three nano-HAPs were conducted under various biogeochemical conditions. Results showed that the fastest U adsorption occurred onto nano-HAP and U adsorption was strongly affected by biogeochemical conditions such as pH and the presence of carbonates, but less affected by temperature. The current study indicates that the presence of carbonates at pH’s above the neutral range in groundwater may inhibit U removal with nanohydroxyapatites.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arey, J. S., Seaman, J. C., & Bertsch, P. M. (1999). Immobilization of uranium in contaminated sediments by hydroxyapatite addition. Environmental Science and Technology, 33, 337–342.
ATSDR (2009). Case Studies in Environmental Medicine (CSEM), Uranium toxicity, May 1, 2009. https://www.atsdr.cdc.gov/csem/uranium/docs/uranium.pdf. Accessed 1 Dec 2020.
Duff, M. C., & Amrhein, C. (1996). Uranium(VI) Adsorption was reported on goethite and soil in carbonate solutions. Soil Science Society of American Journal, 60, 1393–1400.
Fuller, C. C., Bargar, J. R., & Davis, J. A. (2003). Molecular-scale characterization of uranium sorption by bone apatite materials for a permeable reactive barrier demonstration. Environmental Science and Technology, 37(20), 4642–4649.
Fuller, C. C., Bargar, J. R., Davis, J. A., & Piana, M. J. (2002). Mechanisms of uranium interactions with hydroxyapatite implications for groundwater remediation. Environmental Science and Technology, 36, 158–165.
Gorman-Lewis, D., Skanthakumar, S., Jensen, M. P., Mekki, S., Nagy, K. L., & Soderholm, L. (2008). FTIR characterization of amorphous uranyl-silicates. Chemical Geology, 253(3–4), 136–140.
Grunenwald, A., Keyser, C., Sautereau, A., Crubézy, E., Bertrand, L., & Drouet, C. (2014). Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. Journal of Archaeological Science, 49, 134–141.
Gudavalli, R., Katsenovich, Y., & Wellman, D. (2018). Quantification of kinetic rate law parameters for the dissolution of natural autunite in the presence of aqueous bicarbonate ions at high concentrations. Journal of Environmental Radioactivity, 190–191, 1–9.
Hartmann, H. M., Monette, F. A., & Avci, H. L. (2010). Overview of toxicity data and risk assessment methods for evaluating the chemical effects of depleted uranium compounds. Human and Ecological Risk Assessment: An International Journal, 6(5), 851–874.
Jeanjean, J., Rouchaud, J. C., Tran, L., & Fedoroff, M. (1995). Sorption of uranium and other heavy metals on hydroxyapatite. Journal of Radioanalytical and Nuclear Chemistry Letters, 201, 529–539.
Kazery, J. A., Proctor, G., Larson, S. L., Ballard, J. H., Knotek-Smith, H. M., Zhang, Q., Celik, A., Dasari, S., Islam, S. M., Tchounwou, P. B., & Han, F. X. (2021). Distribution and fractionation of uranium in weapon tested range soils. American Chemical Society Earth and Space Chemistry, 5(2), 356–364.
Keith, L.S., Faroon, sO.M., & Fowler, B.A. (2007). Uranium. In: Handbook on the toxicology of metals (3rd Ed, p 881–901). Academic Press.
Kratz, S., & Schnug, E. (2006) Rock phosphates and P fertilizers as sources of U contamination in agricultural soils. In: Merkel B.J., Hasche-Berger A. (eds) Uranium in the Environment. Springer, Berlin, Heidelberg.
Krestou, A., Xenidis, A., & Panias, D. (2004). Mechanism of aqueous uranium(VI) uptake by hydroxyapatite. Minerals Engineering, 17(3), 373–381.
Lammers, L. N., Rasmussen, H., Adilman, D., deLemos, J. L., Zeeb, P., Larson, D. G., & Quicksall, A. N. (2017). Groundwater uranium stabilization by a metastable hydroxyapatite. Applied Geochemistry, 84, 105–113.
Markovic, M., Fowler, B. O., & Tung, M. S. (2004). Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. Journal of Research of the National Institute of Standards and Technology, 109, 553–568.
Martin, W. A., Larson, S. L., Felt, D. R., & Wright, J. (2008). The effect of organics on lead sorption onto apatite II. Applied Geochemistry, 23, 34–43.
Mehta, S. V., Maillot, F., Wang, Z., Catalano, G. J., & Giammar, E. D. (2014). Effect of co-solutes on the products and solubility of uranium (VI) precipitated with phosphate. Chemical Geology, 364, 66–75.
Mehta, S. V., Mailot, F., Wang, Z., Catalano, G. J., & Giammar, E. D. (2015). Transport of U(VI) through sediments amended with phosphate to induce in situ uranium immobilization. Water Research, 69, 307–317.
Newsome, L., Morris, K., & Llyod, R. J. (2014). The biogeochemistry and bioremediation of uranium and other priority radionuclide. Chemical Geology, 363, 164–184.
OECD/NEA. (2001). Management of depleted uranium, nuclear development. OECD Publishing. https://doi.org/10.1787/9789264294998-en
Pan, H., & Darvell, W. B. (2010). Effect of carbonate on hydroxyapatite solubility. Crystal Growth & Design, 10(2), 845–850.
Proctor, G., Larson, S. L., Ballard, J. H., Knotek-smith, H., Waggonor, C., Uzn, R., Li, J., McComb, J., Jin, D., Arslan, Z., & Han, F. X. (2020). Rapid screening uranium in soils using portable X-ray fluorescence spectrometer, a comparative study. ACS Earth & Space Chemistry, 4(2), 211–217.
Ren, F., Ding, Y., & Leng, Y. (2014). Infrared spectroscopic characterization of carbonated apatite: A combined experimental and computational study. Journal of Biomedical Materials Research Part A, 102A, 496–505.
Resende, S. N., Nele, M., & Salim, M. M. V. (2006). Effects of anion substitution on the acid properties of hydroxyapatite. Thermochimica Acta, 451(1–2), 16–21.
Rigali, M. J., Brady, P. V., & Moore, R. C. (2016). Radionuclide removal by apatite. American Mineralogist, 101(12), 2611–2619.
Skwarek, E., Gładysz-Płaska, A., Choromańska, J. B., et al. (2019). Adsorption of uranium ions on nano-hydroxyapatite and modified by Ca and Ag ions. Adsorption, 25, 639–647.
Slosarczyk, A., Paszkiewicz, Z., & Paluszkiewicz, C. (2005). FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. Journal of Molecular Structure, 744–747, 657–661.
Soudek, P., Petrova, S., Benesova, D., Dvorakova, M., & Vanek, T. (2011). Uranium uptake by hydroponically cultivated crop plants. Journal of Environmental Radioactivity, 102(6), 598–604.
Stewart, B. D., Mayes, M. A., & Fendorf, S. (2010). Impact of uranyl−calcium−carbonate complexes on uranium(VI) adsorption to synthetic and natural sediments. Environmental Science and Technology, 44(3), 928–934.
Wang, M., Zhang, K., Wu, M., Wu, Q., Liu, J., Yang, J., & Zhang, J. (2019). Unexpectedly high adsorption capacity of esterified hydroxyapatite for heavy metal removal. Langmuir, 35(49), 16111–16119.
Yang, D., Wang, X., Song, G., Zhao, G., Chen, Z., Yu, S., Gu, P., Wang, H., & Wang, X. (2017). One-pot synthesis of arginine modified hydroxyapatite carbonate microsphere composites for efficient removal of U(VI) from aqueous solutions. Science Bulletin, 62, 1609–1618.
Yuan, Y., Liu, T., Xiao, J., et al. (2020). DNA nano-pocket for ultra-selective uranyl extraction from seawater. Nature Communication, 11, 5708.
Zhang, D., Chen, X., Larson, S. L., Ballard, J. H., Knotek-Smith, H. M., Ding, D., Hu, N., & Han, F. X. (2020b). Uranium biomineralization with phosphate - biogeochemical process and its application. American Chemical Society Earth and Space Chemistry, 4(12), 2205–2214.
Zhang, Q., Larson, S. L., Ballard, J. H., Zhu, X., Knotek-Smith, H. M., & Han, F. X. (2020a). Uranium metal corrosion in soils with different soil moisture regimes. Corrosion Science, 179, 109138.
Zhou, P., & Gu, B. (2005). Extraction of oxidized and reduced forms of uranium from contaminated soils: Effects of carbonate concentration and pH. Environmental Science & Technology, 39, 4435–4440.
Funding
This study was supported by the U.S. Army Engineer Research and Development Center (W912HZ-16–2-0021), the US Nuclear Regulatory Commission (NRC-HQ-84–16-G-0040, NRC-HQ-84–15-G-0042 and NRC–HQ-12-G-38–0038), and the US Department of Commerce (NOAA) (NA11SEC4810001-003499, NA16SEC4810009, NOAA Center for Coastal and Marine Ecosystems Grant # G634C22).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cooper, P., Nie, J., Larson, S.L. et al. Uranium Adsorption on Three Nanohydroxyapatites Under Various Biogeochemical Conditions. Water Air Soil Pollut 232, 362 (2021). https://doi.org/10.1007/s11270-021-05298-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05298-7