Abstract
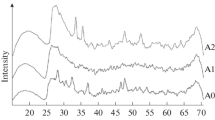
The effect of a magnetic field on the crystallisation and morphology of CaCO3 in solution was investigated in the 25–80 °C temperature range. The samples were analysed using X-ray diffraction, FTIR, SEM analysis and quantitative analysis based on the Rietveld method. It was demonstrated that the magnetic field, which varied from 50 to 175 mT, had an impact on the polymorphs selectivity and their proportions and on the morphology and the sizes of the CaCO3 particles. It also reduced the overall precipitation rate of CaCO3 minerals in the solution. SEM images showed that the magnetic field modifies the shape of the rhombs of calcite as reflected by the destruction of the particles at 25 and 50 °C with a considerable decrease in their size at 80 °C. These results demonstrate the effectiveness of a magnetic field in reducing the adhesion of calcite to surfaces and thus its possible application in limiting the formation of scale in industrial installations.










Similar content being viewed by others
References
A. Fathi et al., Effect of a magnetic water treatment on homogeneous and heterogeneous precipitation of calcium carbonate. Water Res. 40(10), 1941–1950 (2006)
E. Chibowski, A. Szczes, L. Holysz, Influence of sodium dodecyl sulfate and static magnetic field on the properties of freshly precipitated calcium carbonate. Langmuir 21(18), 8114–8122 (2005)
S. Kobe et al., The influence of the magnetic field on the crystallisation form of calcium carbonate and the testing of a magnetic water-treatment device. J. Magn. Magn. Mater. 236(1–2), 71–76 (2001)
M. Gryta, The influence of magnetic water treatment on CaCO3 scale formation in membrane distillation process. Sep. Purif. Technol. 80(2), 293–299 (2011)
C.Y. Tai et al., Magnetic effects on crystal growth rate of calcite in a constant-composition environment. J. Cryst. Growth 310(15), 3690–3697 (2008)
A. Szkatula, M. Balanda, M. Kopeć, Magnetic treatment of industrial water. Silica activation. Eur. Phys. J.-Appl. Phys. 18(1), 41–49 (2002)
L. Lipus, D.J.C.E.S. Dobersek, Influence of magnetic field on the aragonite precipitation. Chem. Eng. Sci. 62(7), 2089–2095 (2007)
K. Higashitani et al., Effects of a magnetic field on the formation of CaCO3 particles. J. Colloid Interface Sci. 156(1), 90–95 (1993)
R.A. Barrett, S.A. Parsons, The influence of magnetic fields on calcium carbonate precipitation. Water Res. 32(3), 609–612 (1998)
L. Hołysz, M. Chibowski, E. Chibowski, Time-dependent changes of zeta potential and other parameters of in situ calcium carbonate due to magnetic field treatment. Colloids Surf., A 208(1–3), 231–240 (2002)
C. Gabrielli et al., Magnetic water treatment for scale prevention. Water Res. 35(13), 3249–3259 (2001)
N.V.-Y. Quach, A. Li, J.C. Earthman, Interaction of calcium carbonate with nanobubbles produced in an alternating magnetic field. ACS Appl. Mater. Interfaces. 12(39), 43714–43719 (2020)
F. Amrouche et al., Effect of magnetic field on physiochemical properties of carbonate reservoirs. in 82nd EAGE Annual Conference & Exhibition. European Association of Geoscientists & Engineers. 2020(1), 1–5 (2020)
R. Mghaiouini et al., The electromagnetic memory of water at kinetic condition. Int. J. Curr. Eng. Technol. 10(1), 11 (2020)
Z. Lv et al., Analysis of factors affecting magnetic memory tme of CaCO3 solutions based on orthogonal experiment. Sensors Materials 32(4), 1339–1350 (2020)
S. Knez, C. Pohar, The magnetic field influence on the polymorph composition of CaCO3 precipitated from carbonized aqueous solutions. J. Colloid Interface Sci. 281(2), 377–388 (2005)
A. Szcześ et al., Effects of static magnetic field on water at kinetic condition. Chem. Eng. Process. 50(1), 124–127 (2011)
E. Chibowski, L. Hołysz, A. Szcześ, Adhesion of in situ precipitated calcium carbonate in the presence and absence of magnetic field in quiescent conditions on different solid surfaces. Water Res. 37(19), 4685–4692 (2003)
R. Gehr et al., Reduction of soluble mineral concentrations in CaSO4 saturated water using a magnetic field. Water Res. 29(3), 933–940 (1995)
H.L. Madsen, Influence of magnetic field on the precipitation of some inorganic salts. J. Cryst. Growth 152(1–2), 94–100 (1995)
J. Coey, S. Cass, Magnetic water treatment. J. Magn. Magn. Mater. 209(1–3), 71–74 (2000)
S. Sutomo et al., The morphology aspect of CaCO3 scale impact of solenoid magnetic field in the various flow rate solution in the piping system. Materials Today: Proceedings 13, 287–292 (2019)
C. Sronsri, U. Kongpop, W. Sittipol, Quantitative analysis of calcium carbonate formation in magnetized water. Mater. Chem. Phys. 245, 122735–122744 (2020)
L. Lutterotti, S. Matthies, H. Wenk, MAUD: a friendly Java program for material analysis using diffraction. IUCr: Newsletter of the CPD. 21, 14–15 (1999)
Z.G. Wu et al., Preparation of vaterite CaCO3 microspheres by fast precipitation method. Int. J. Mater. Res. 108(3), 245–248 (2017)
R. Ševčík et al., Characterization of vaterite synthesized at various temperatures and stirring velocities without use of additives. Powder Technol. 284, 265–271 (2015)
S. Gopi, V. Subramanian, K. Palanisamy, Aragonite–calcite–vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bull. 48(5), 1906–1912 (2013)
L. Gránásy et al., Phase field theory of crystal nucleation and polycrystalline growth: A review. J. Mater. Res. 21(2), 309–319 (2006)
J.-P. Andreassen, Formation mechanism and morphology in precipitation of vaterite—nano-aggregation or crystal growth? J. Cryst. Growth 274(1–2), 256–264 (2005)
K.K. Sand et al., Crystallization of CaCO3 in water–alcohol mixtures: spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth Des. 12(2), 842–853 (2011)
J.Q. Qi et al., Electric field-controlled crystallizing CaCO3 nanostructures from solution. Nanoscale Res. Lett. 11(1), 120 (2016)
K.K. Sand et al., Crystallization of CaCO3 in water–alcohol mixtures: spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth Des. 12(2), 842–853 (2012)
A. Alabi et al., Advances in anti-scale magnetic water treatment. Environ. Sci.: Water Res. Technol. 1(4), 408–425 (2015)
J. Coey, S. Cass, Magnetic water treatment. J. Magn. Magn. Mater. 209(1–3), 71–74 (2000)
U. Ojaniemi et al., Wall function model for particulate fouling applying XDLVO theory. Chem. Eng. Sci. 84, 57–69 (2012)
P. Moulin, H. Roques, Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 261(1), 115–126 (2003)
Acknowledgements
Authors acknowledge the University of Bejaia and la Direction Générale de la Recherche Scientifique et du Développement Technologique (DGRSDT) for funding. This work was achieved in the framework of the PRFU project No. B00L02UN060120180005.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing for financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amer, L., Ouhenia, S., Chateigner, D. et al. The effect of a magnetic field on the precipitation of calcium carbonate. Appl. Phys. A 127, 716 (2021). https://doi.org/10.1007/s00339-021-04860-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04860-8