Published online Aug 6, 2021. doi: 10.12998/wjcc.v9.i22.6493
Peer-review started: April 7, 2021
First decision: April 23, 2021
Revised: April 26, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: August 6, 2021
Phlegmonous gastritis (PG) is a rare bacterial infectious disease characterized by neutrophil-based purulent inflammation of the gastric wall. The most representative causative bacterium is Streptococcus pyogenes, followed by Staphylococcus, Pneumococcus and Enterococcus. Hepatic portal venous gas (HPVG) is considered a potentially fatal condition and is rarely associated with PG.
The white blood cell count of a 70-year-old woman with acute lymphocytic leukemia in complete remission dropped to 100/μL after consolidation chemotherapy. Her vital signs were consistent with septic shock. Venous blood culture revealed the presence of Bacillus cereus. Abdominal computed tomography (CT) and esophagogastroduodenoscopy (EGD) showed marked thickening of the gastric wall. As with the other findings, CT was suggestive of HPVG, and EGD showed pseudomembrane-like tissue covering the superficial mucosa. Histopathological examination of gastric biopsy specimens showed mostly necrotic tissue with lymphocytes rather than neutrophils. Culture of gastric specimens revealed the presence of Bacillus cereus. We finally diagnosed this case as PG with Bacillus cereus-induced sepsis and HPVG. This patient recovered successfully with conservative treatment, chiefly by using carbapenem antibiotics.
The histopathological finding of this gastric biopsy specimen should be called "neutropenic necrotizing gastritis".
Core Tip: We reported a case of phlegmonous gastritis due to Bacillus cereus infection during the neutropenic phase after consolidation chemotherapy for acute lymphocytic leukemia. Including our 2 patients, we analyzed 7 similar patients reported in the past. Histopathological examination with gastric biopsy was performed only in our two patients, and in both cases, characteristic findings that should be called "neutropenic necrotizing gastritis" were observed.
- Citation: Saito M, Morioka M, Izumiyama K, Mori A, Ogasawara R, Kondo T, Miyajima T, Yokoyama E, Tanikawa S. Phlegmonous gastritis developed during chemotherapy for acute lymphocytic leukemia: A case report. World J Clin Cases 2021; 9(22): 6493-6500
- URL: https://www.wjgnet.com/2307-8960/full/v9/i22/6493.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i22.6493
Phlegmonous gastritis (PG) is a rare bacterial infectious disease characterized by neutrophil-based purulent inflammation of the gastric wall starting from the submucosa and muscularis layers[1]. In approximately 70% of causes, the causative bacterium is Streptococcus pyogenes, and other causative bacteria include Staphylococcus, Pneumococcus and Enterococcus[2]. Alcoholism, mucosal injury, gastric hemorrhage and immunosuppression have been reported to be risk factors for PG[2,3]. PG is regarded as a disease with a high mortality rate of approximately 40%, even if treated in a timely manner[3,4]. There are only a few reports on PG with hematological malig
Previously, we reported a case of PG with Bacillus cereus-induced sepsis in which the neutrophil count was markedly reduced during chemotherapy for acute lymphocytic leukemia (ALL)[9]. In this study, we report a case of a similar pathological condition accompanied by HPVG followed by multiple liver abscesses.
A 70-year-old woman complained of low-grade fever (37.5 °C), general fatigue, loss of appetite for 2 d, and nausea and watery diarrhea beginning on day 11 (with day 1 being the day on which chemotherapy was started).
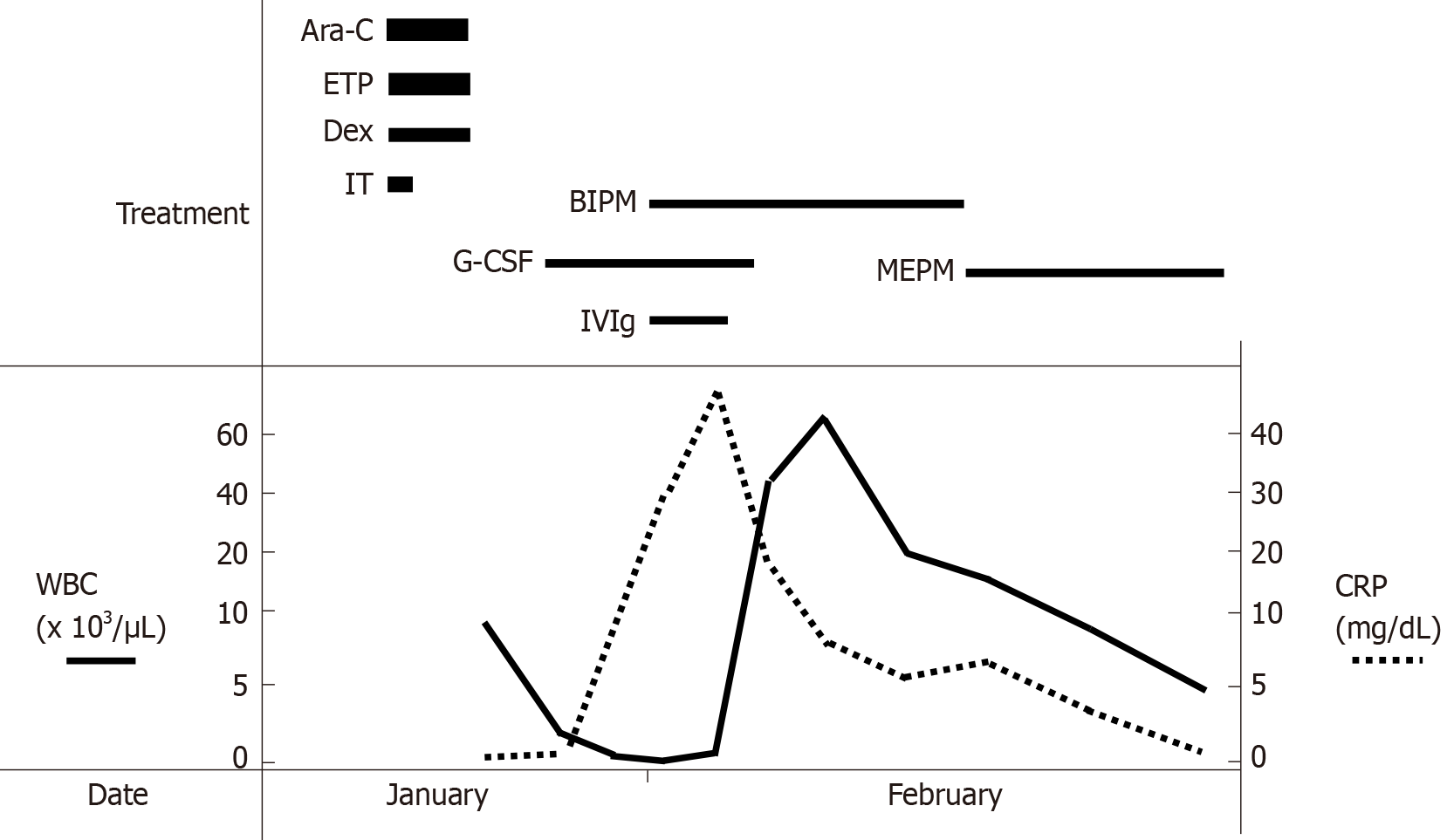
Four months ago, she had developed ALL and achieved complete remission (CR) with induction chemotherapy. She received consolidation chemotherapy with cytarabine (1.4 g, 2 times/d), etoposide (100 mg/d) and dexamethasone (33 mg/d) for 3 d (Figure 1). On the first day (day 1), intrathecal injection of methotrexate (15 mg), cytarabine (40 mg) and prednisolone (10 mg) was also administered. The white blood cell count (WBC) began to decrease steadily, and on day 7, a granulocyte-colony stimulating factor (G-CSF) preparation (lenograstim) was used, but the decline continued.
She was on medication for dyslipidemia and gastroesophageal reflux disease. For the latter, she was taking vonoprazan (10 mg) before she developed the present disease.
There was no personal and family history.
On day 11, she exhibited the following vital signs that were consistent with septic shock: body temperature increase to 38.1 °C; temporary drop in blood pressure to 78/60 mmHg; heart rate of 126 beats/min; respiratory rate of 31 breaths/min; and pulse oximetry (SpO2) of 96%. She was obviously sick and was lying down, but there was no apparent loss of consciousness. Physical examination revealed mild tenderness in the upper abdomen.
The venous blood culture was later found to reveal the presence of Bacillus cereus. The culture of the tip of the removed central venous catheter was negative. The other data on day 11 to day 17 are shown in Table 1. WBC had decreased to 100/μL on day 11 (also shown in Figure 1). C-reactive protein (CRP) levels increased rapidly to 29.71 mg/dL on day 11 and to 46.82 mg/dL on day 13 (Figure 1). WBC markedly increased to 45000/μL on day 15 and 66000/μL on day 17 (Figure 1) despite stopping G-CSF. Liver dysfunction was observed in conjunction with elevated WBC.
| (Normal range) | Day 11 | Day 13 | Day 15 | Day 17 | |
| WBC (/μL) | (4000-8000) | 100 | 500 | 45000 | 66000 |
| Myelocyte | - | 1 | 10 | 7 | |
| Metamyelocyte | - | 1 | 4 | 14 | |
| Stab leukocytes (%) | 0-6 | 3 | 3 | 4 | |
| Segmented leukocytes (%) | 45-68 | 98 | 49 | 59 | 67 |
| Lymphocytes (%) | 20-45 | 1 | 16 | 5 | 1 |
| Monocytes (%) | 2-8 | 1 | 29 | 19 | 7 |
| Eosinophils (%) | 0-6 | 1 | |||
| RBC (× 104/μL) | 380-500 | 448 | 260 | 303 | 318 |
| Hb (g/dL) | 12.0-16.0 | 11.5 | 6.7 | 8.1 | 8.6 |
| Platelet (× 104/μL) | 12.0-40.0 | 3.2 | 3.3 | 8.1 | 17.9 |
| CRP (mg/dL) | < 0.30 | 29.71 | 46.82 | 16.93 | 8.01 |
| TP (g/dL) | 6.7-8.3 | 5.1 | 4.2 | 4.1 | 4.3 |
| Alb (g/dL) | 3.8-5.3 | 2.9 | 1.9 | 1.9 | 2.2 |
| AST (U/L) | 8-38 | 41 | 21 | 67 | 48 |
| ALT (U/L) | 4-44 | 34 | 26 | 79 | 79 |
| LDH (U/L) | 120-245 | 142 | 299 | 1175 | 973 |
| ALP (U/L) | 105-330 | 215 | 232 | 388 | 545 |
| γ-GTP (U/L) | < 30 | 15 | 13 | 43 | 72 |
| T-Bil (mg/dL) | 0.2-1.2 | 0.5 | 0.5 | 0.4 | 0.4 |
| BUN (mg/dL) | 8.0-20.0 | 39.4 | 21.2 | 17.8 | 14.7 |
| Cr (mg/dL) | 0.50-0.86 | 1.97 | 1.11 | 0.77 | 0.85 |
| e-GFR (mL/min/1.73 m2) | > 90 | 20.2 | 38.2 | 56.4 | 50.6 |
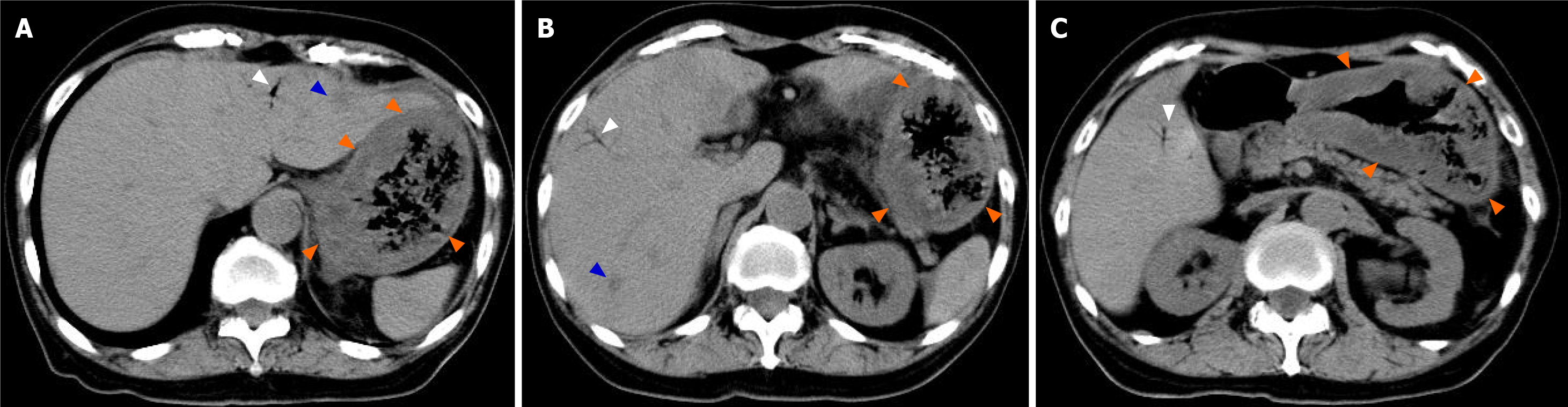
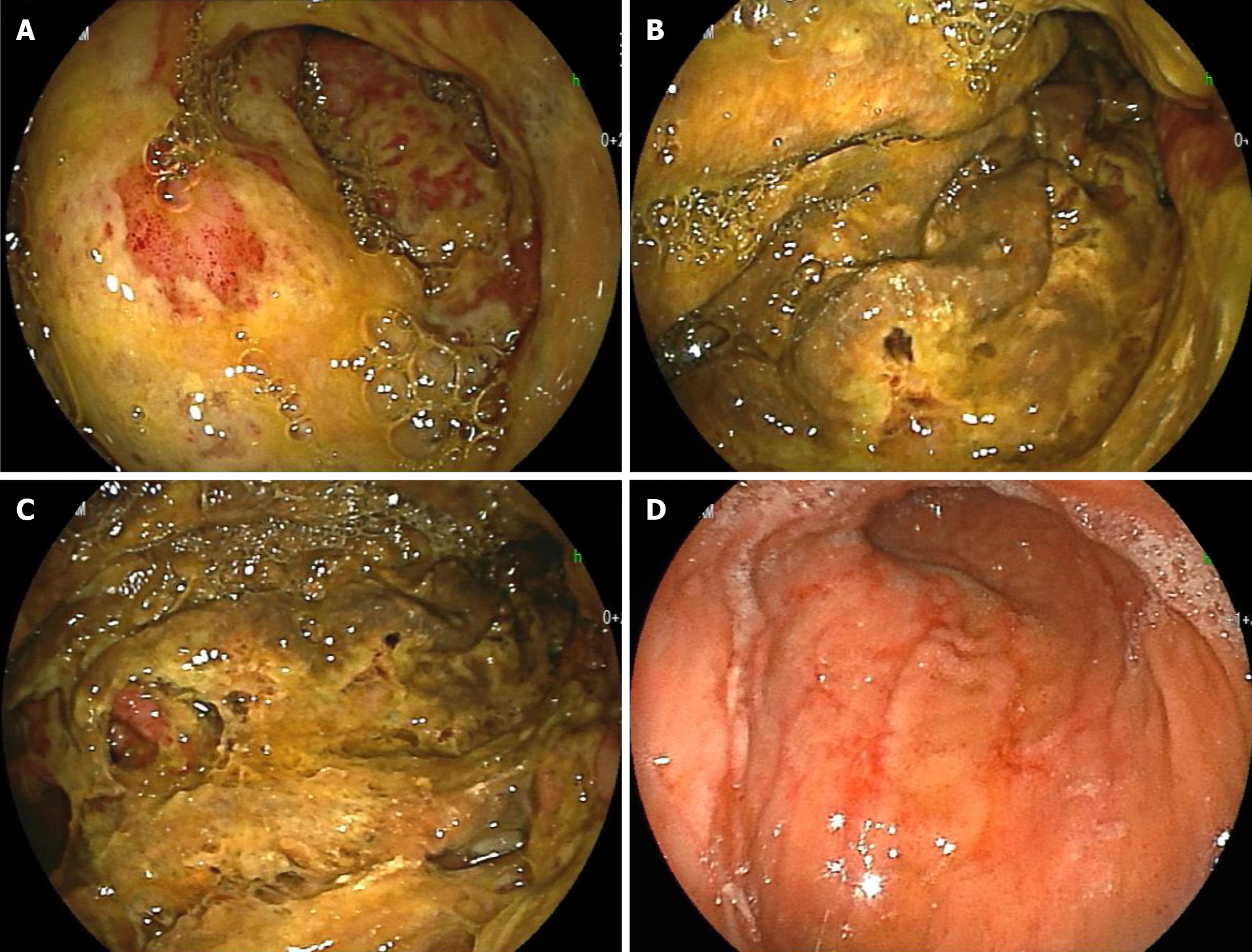
An abdominal computed tomography (CT) scan on day 11 showed marked thickening of the gastric wall (Figure 2, orange arrow). CT also showed findings suggestive of HPVG scattered in the liver (Figure 2, white arrow); in addition, low-density areas (LDAs) were found in liver S3 and S7 (Figure 2, blue arrow). Esophagogastroduodenoscopy (EGD) on day 14 showed marked thickening of the gastric wall in the corpus of the stomach as well as yellow-green pseudomembrane-like tissue covering the superficial mucosa (Figure 3A-C). This patient was clinically diagnosed with PG with HPVG.
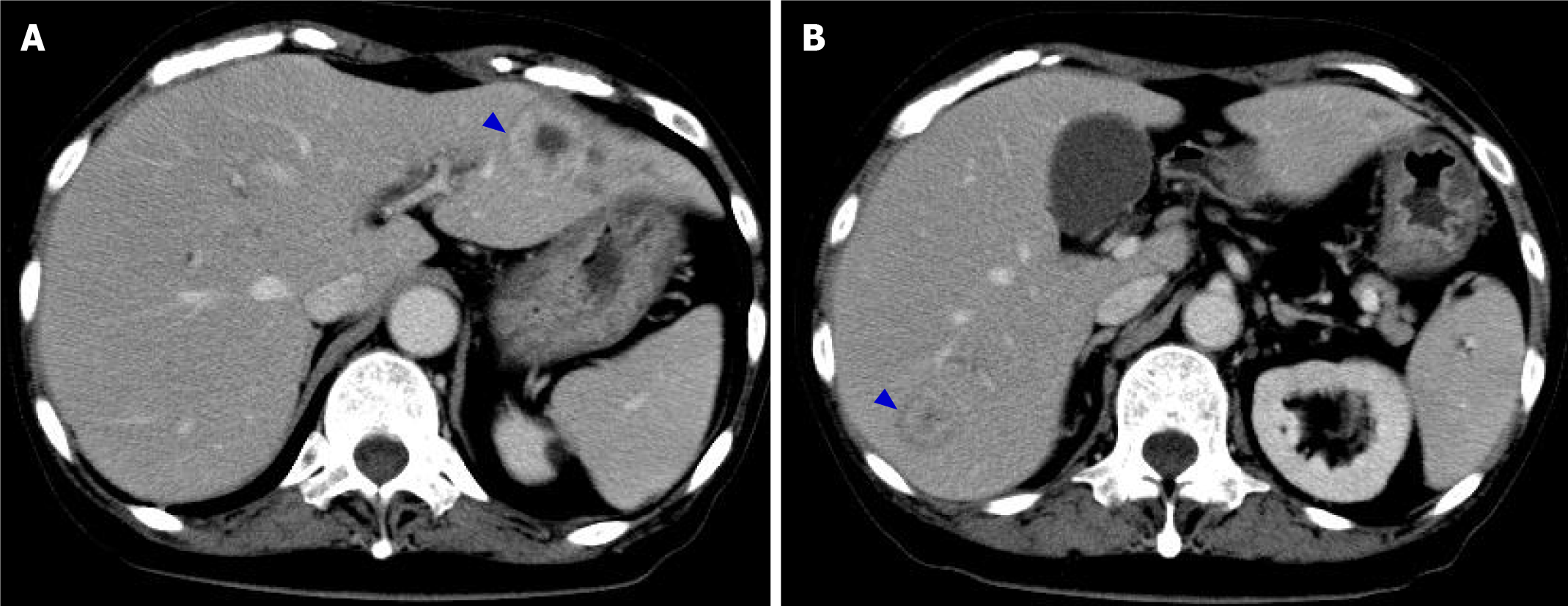
EGD on day 29 revealed that the abovementioned abnormal findings improved in 15 d, and linear redness, erosion and ulcerative mucosal changes were observed (Figure 3D). On the same day, CT showed improvements in thickening of the gastric wall, and the findings suggestive of HPVG disappeared (Figure 4). In addition, LDAs in liver S3 and S7 originally observed on day 11 changed to findings consistent with abscesses (Figure 4, blue arrow).
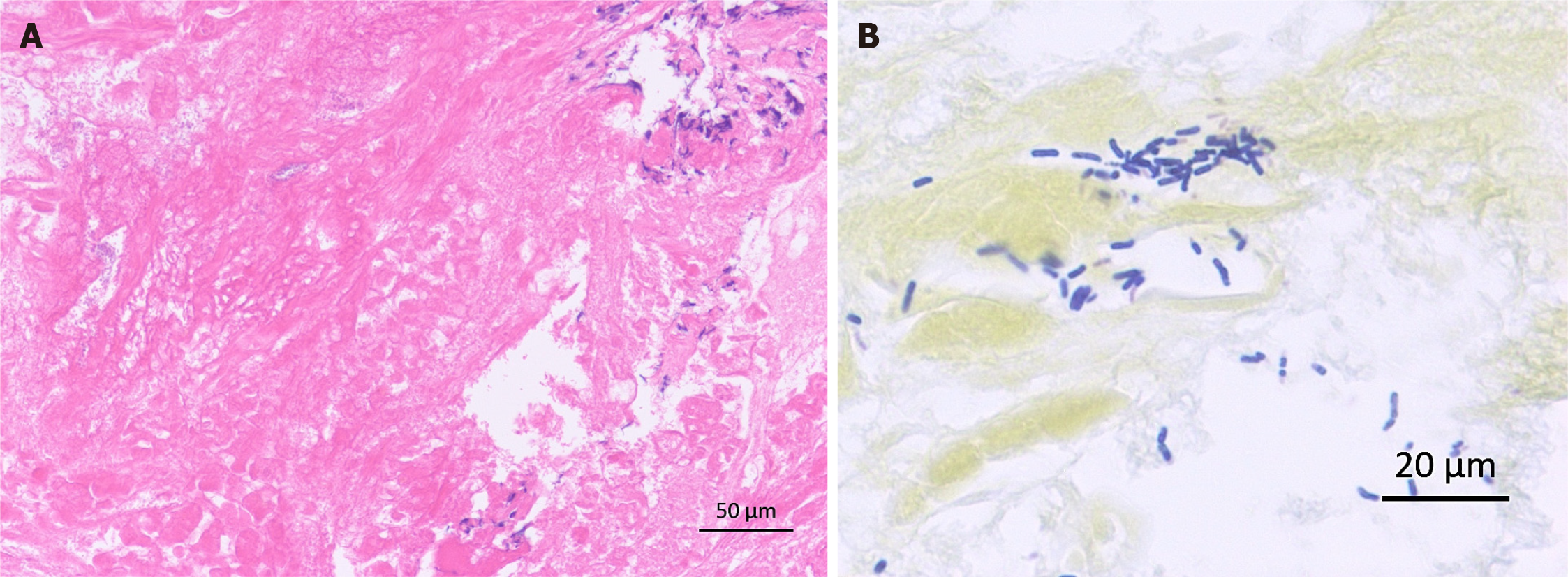
Histopathological analysis of gastric biopsy specimens showed mostly necrotic tissue with fibrin precipitation and partial infiltration of inflammatory cells by lymphocytes rather than neutrophils (Figure 5A). It was unique findings that should be called "neutropenic necrotizing gastritis". Bacilli showing positive Gram staining were also observed (Figure 5B). Culture of gastric specimens later proved that this bacterial group was Bacillus cereus. We finally diagnosed this case as PG with Bacillus cereus-induced sepsis and HPVG followed by multiple liver abscesses. The causative bacteria of the liver abscesses were not investigated.
The clinical course of this patient is shown in Figure 1. After day 11, while use of G-CSF was continued for severe infection, administration of carbapenem antibiotics (initially biapenem 0.3 g, 2 times/d and then meropenem 0.5 g, 2 times/d) was used in combination with intravenous immunoglobulin (5 g/d) for 3 d. In addition to antibiotics, septic shock was treated by administration of hydrocortisone and fluid. Red blood cells and platelets were transfused once each. No invasive treatment was administered for liver abscess.
This patient recovered successfully with conservative treatment, chiefly administration of antibiotics, without any sequelae. She then received intrathecal injection twice and repeated consolidation therapy for ALL with blinatumomab (a CD19/CD3 bispecific T-cell engager) for 3 cycles. She has no recurrence of PG more than one year and is maintaining CR for ALL. Currently, she is being followed-up with no treatment as an outpatient.
Our patient with ALL developed PG due to Bacillus cereus infection during the neutrophil depletion period after consolidation chemotherapy and was temporarily in a fatal state of septic shock. We reported a similar case in 2012 in which severe inflammation, such as an increase in CRP levels to 52.97 mg/dL, was observed, and we were able to save the lives of patient at that time[9] (since then, the patient has been able to lead a completely normal daily life for more than 11 years). Regarding the current patient, on the day that WBC dropped to 100/μL, the patient complained of gastro
In general, hematological malignancies tend to be accompanied by a decrease in immunocompetence, and myelosuppression by the administration of anticancer drugs further promotes immunodeficiency. There have been 6 reports of patients with hematological malignancies who developed PG during the neutropenic phase after the administration of anticancer drugs, including 1 of our previous patients[9-14]. Table 2 summarizes the clinical features of a total of 7 patients, including the patient experienced this time. Notably, Enterococcus, which is one of the representative causative bacterial species of PG, was detected in only 2 cases, whereas Bacillus, which has rarely been reported as a causative agent of PG, was detected in 4 cases, including our 2 cases. Neutrophil-based purulent inflammation of the gastric wall is considered to be the main cause of PG, whereas in 5 of the 7 cases, the neutrophil count was reduced to less than 100/μL. It was presumed that there is a difference in the onset process as a bacterial infectious disease. Of 7 cases, only our two cases were histopathologically examined with gastric biopsy, and unlike typical PG findings, most were covered with necrotic tissue, in which gram-positive bacilli were found. Infiltration of inflammatory cells by lymphocytes but not neutrophils was partly observed. This finding should be called "neutropenic necrotizing gastritis", which has never been proposed, rather than the typical PG. Bacillus, one of the residents of the bacterial flora in the small intestine, infected the gastric wall under immunosuppression and caused PG in this patient. Bacillus cereus produces various toxins[15], including necrotizing enterotoxin which may have been present in our two patients.
| No. | Ref. | Age | Sex | Hematological malignancy | WBC/neutrophils | Pathogen(s) | Histopathological examination | Gastric acid secretion inhibitor | Outcome |
| 1 | Takeuchi et al[10] | 63 | M | ATLL | 300/unknown | Gram-positive cocci and Gram-negative bacilli1 | Not executed | H2-RA | Death |
| 2 | Matsumoto et al[11] | 74 | M | MF and MM | 800/300 | Bacillus thuringiensis | Not executed | Unknown | Death |
| 3 | Iqbal et al[12] | 59 | F | AML (2nd relapse) | < 100/0 | Citrobacter freundii/Enterococcus faecalis/Bacillus cereus | Not executed | Unknown | Recovered |
| 4 | Inagawa et al[13] | 34 | F | APL with MF | 320/20 | Enterobacter cloacae/Enterococcus species/MRS | Not executed | PPI | Death |
| 5 | Shi et al[14] | 33 | M | MPAL | 70/unknown | Stenotrophomonas maltophilia | Not executed | PPI | Recovered |
| 6 | Saito et al[9] | 55 | F | ALL | 100/4 | Bacillus cereus | Executed | PPI | Recovered |
| 7 (this case) | 70 | F | ALL | 100/98 | Bacillus cereus | Executed | P-CAB | Recovered |
Gastric acid secretion inhibitors were used in 5 cases (two cases were not described). Recently, it was reported that the risk of intestinal infectious diseases such as Clostridium difficile is increased by disruption of the function of the barrier that prevents the invasion of pathogens into the gastrointestinal tract by a gastric acid secretion inhibitor[16]. It was reported that vonoprazan, a potassium-competitive acid blocker used in this patient, showed a stronger gastric acid secretion inhibitory effect than proton pump inhibitor and induced a stronger change in the composition of the intestinal flora[17]. Shi et al[14] expressed concern that the use of gastric acid secretion inhibitors increases the bacterial abundance in the gastric juice and thus may result in risk for the development of PG. Currently, there are few patients with PG with or without hematological malignancies, and scientific evidence is limited. In the future, a large number of patients will need to be aggregated for further study.
This patient also developed HPVG and liver abscess. HPVG was previously a sign of poor prognosis, but in recent years, the mortality rate of patients with HPVG detected by CT has been lower (29%) than that previously reported, and HPVG alone cannot predict prognosis[18]. In our case, a small amount of HPVG was found in the initial CT that suggested PG, and we were able to conservatively respond before it spread. In addition, formation of liver abscess could be followed by CT from the beginning. We have not been able to identify the bacterial species responsible for the liver abscess; however, we believe that the causative agent was Bacillus cereus, which spread through the portal vein. Furthermore the liver abscesses could also be conservatively cured without drainage.
We reported a case of PG due to Bacillus cereus infection during the neutropenic phase after the administration of anticancer drugs for ALL. We analyzed 7 cases with similar pathological conditions reported previously, including the 2 cases we experienced. The histopathological findings in our two patients should be called "neutropenic necrotizing gastritis", which has never been proposed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tang SH S-Editor: Yan JP L-Editor: A P-Editor: Wang LYT
| 1. | Kato K, Tominaga K, Sugimori S, Nagami Y, Kamata N, Yamagami H, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Arakawa T. Successful Treatment of Early-Diagnosed Primary Phlegmonous Gastritis. Intern Med. 2015;54:2863-2866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Rada-Palomino A, Muñoz-Duyos A, Pérez-Romero N, Vargas-Pierola H, Puértolas-Rico N, Ruiz-Campos L, Espinós-Pérez J, Veloso-Veloso E. Phlegmonous gastritis: A rare entity as a differential diagnostic of an acute abdomen. Description of a case and a bibliographic review. Rev Esp Enferm Dig. 2014;106:418-424. [PubMed] [Cited in This Article: ] |
| 3. | Kim GY, Ward J, Henessey B, Peji J, Godell C, Desta H, Arlin S, Tzagournis J, Thomas F. Phlegmonous gastritis: case report and review. Gastrointest Endosc. 2005;61:168-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Park CW, Kim A, Cha SW, Jung SH, Yang HW, Lee YJ, Lee HIe, Kim SH, Kim YH. A case of phlegmonous gastritis associated with marked gastric distension. Gut Liver. 2010;4:415-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Harikumar R, Pramod K, Pushpa M, Simi K, Arun G. Gastric lymphoma presenting as phlegmonous gastritis. J Gastrointest Cancer. 2007;38:24-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Guo J, Young SK, Lorenzo CR, Lee CM, Kanel GC, Brynes RK, Chandrasoma P, Naritoku WY. Phlegmonous gastritis in a patient with myeloid sarcoma: a case report. Appl Immunohistochem Mol Morphol. 2009;17:458-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Liu YJ, Siracuse JJ, Gage T, Hauser CJ. Phlegmonous gastritis presenting as portal venous pneumatosis. Surg Infect (Larchmt). 2013;14:221-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Yasuda T, Yagi N, Nakahata Y, Kurobe T, Yasuda Y, Omatsu T, Obora A, Kojima T. A case of phlegmonous gastritis with hepatic portal venous gas caused by Aeromonas hydrophila successfully treated with medication. Clin J Gastroenterol. 2020;13:281-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Saito M, Morioka M, Kanno H, Tanaka S. Acute phlegmonous gastritis with neutropenia. Intern Med. 2012;51:2987-2988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Takeuchi M, Uno H, Matsuoka H, Maeda K, Marutsuka K, Sumiyoshi A, Tsuda K, Tsubouchi H. [Acute necrotizing gastritis associated with adult T-cell leukemia in the course of chemotherapy]. Gan To Kagaku Ryoho. 1995;22:289-292. [PubMed] [Cited in This Article: ] |
| 11. | Matsumoto H, Ogura H, Seki M, Ohnishi M, Shimazu T. Fulminant phlegmonitis of the esophagus, stomach, and duodenum due to Bacillus thuringiensis. World J Gastroenterol. 2015;21:3741-3745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Iqbal M, Saleem R, Ahmed S, Jani P, Alvarez S, Tun HW. Successful Antimicrobial Treatment of Phlegmonous Gastritis: A Case Report and Literature Review. Case Rep Hematol. 2018;2018:8274732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 13. | Inagawa Y, Komeno Y, Saito S, Maenohara Y, Yamagishi T, Kawashima H, Saito T, Abe K, Iihara K, Hatada Y, Ryu T. Prolonged Myelosuppression due to Progressive Bone Marrow Fibrosis in a Patient with Acute Promyelocytic Leukemia. Case Rep Hematol. 2019;2019:1616237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shi D, He J, Lv M, Liu R, Zhao T, Jiang Q. Phlegmonous gastritis in a patient with mixed-phenotype acute leukemia in the neutropenia phase during chemotherapy: A case report. Medicine (Baltimore). 2019;98:e17777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 763] [Cited by in F6Publishing: 623] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 16. | Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 393] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Otsuka T, Sugimoto M, Inoue R, Ohno M, Ban H, Nishida A, Inatomi O, Takahashi S, Naito Y, Andoh A. Influence of potassium-competitive acid blocker on the gut microbiome of Helicobacter pylori-negative healthy individuals. Gut. 2017;66:1723-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Faberman RS, Mayo-Smith WW. Outcome of 17 patients with portal venous gas detected by CT. AJR Am J Roentgenol. 1997;169:1535-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |