Abstract
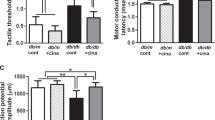
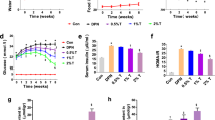
Diabetic peripheral neuropathy (DPN) is a chronic complication of diabetes, and its neural mechanisms underlying the pathogenesis remain unclear. Autophagy plays an important role in neurodegenerative diseases and nerve tissue injury. Lipin1 is a phosphatidic acid phosphatase enzyme that converts phosphatidic acid (PA) into diacylglycerol (DAG), a precursor of triacylglycerol and phospholipids which plays an important role in maintaining normal peripheral nerve conduction function. However, whether Lipin1 involved in the pathogenesis of DPN via regulation of autophagy is not elucidated. Here, we show that the Lipin1 expression was downregulated in streptozotocin (STZ)-induced DPN rat model. Interestingly, STZ prevented DAG synthesis, and resulted in autophagic hyperactivity, effects which may increase the apoptosis of Schwann cells and lead to demyelination in sciatic nerve in DPN rats. More importantly, upregulation of lipin1 in the DPN rats ameliorated autophagy disorders and pathological changes of the sciatic nerve, which associated with the increase of the motor nerve conductive velocity (MNCV) in DPN rats. In contrast, knockdown of lipin1 exacerbates neuronal abnormalities and facilitates the genesis of DPN phenotypes in rats. In addition, overexpression of lipin1 in RSC96 cells also significantly decreased the autophagic hyperactivity and apoptosis induced by hyperglycemia. These results suggest that lipin1 may exert neuroprotection within the sciatic nerve anomalies and may serve as a potential therapeutic target for the treatment of DPN.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and are available from the corresponding author upon reasonable request.
Abbreviations
- 7-AAD:
-
7-Aminoactinomycin D
- ADV:
-
Adenovirus
- ADVs:
-
Adenoviral vectors
- BCA:
-
Bicinchoninic acid
- CCK-8:
-
Cell counting kit-8
- DAG:
-
Diacylglycerol
- DAPI:
-
4′,6-Diamidine-2-phenylidole dihydrochloride
- DM:
-
Diabetes mellitus
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DPN:
-
Diabetic peripheral neuropathy
- ELISA:
-
Enzyme-linked immunosorbent assay
- EM:
-
Electron microscopy
- FBS:
-
Fetal bovine serum
- FBG:
-
Fasting-blood-glucose
- FITC:
-
Fluorescein isothiocyanate
- LV:
-
Lentivirus
- LVs:
-
Lentiviral vectors
- MNCV:
-
Motor nerve conduction velocity
- MOI:
-
Multiplicity of infection
- NC:
-
Negative control
- PA:
-
Phosphatidic acid
- PAP:
-
Phosphatidic acid phosphatase
- PBS:
-
Phosphate-buffered saline
- PKD:
-
Protein kinase D
- PMWT:
-
Paw mechanical withdrawal threshold
- PVDF:
-
Polyvinylidene fluoride
- qPCR:
-
Quantitative real-time polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- STZ:
-
Streptozotocin
- TBS-T:
-
Tris-buffered saline Tween
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, IDF Diabetes Atlas Committee (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843
Kang C, LeRoith D, Gallagher EJ (2018) Diabetes, obesity, and breast cancer. Endocrinology 159(11):3801–3812
Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. Lancet 389(10085):2239–2251
Lin S, Rocha VM, Taylor R (2019) Artefactual inflation of type 2 diabetes prevalence in WHO STEP surveys. Trop Med Int Health 24(4):477–483
Tesfaye S, Selvarajah D, Gandhi R et al (2016) Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging. Pain 157(Suppl 1):S72–S80
Alleman CJ, Westerhout KY, Hensen M et al (2015) Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res Clin Pract 109(2):215–225
Sloan G, Shillo P, Selvarajah D et al (2018) A new look at painful diabetic neuropathy. Diabetes Res Clin Pract 144:177–191
Tesfaye S, Boulton AJ, Dickenson AH (2013) Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 36(9):2456–2465
Feldman EL, Nave KA, Jensen TS, Bennett DL (2017) New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 93(6):1296–1313
Ghavami S, Shojaei S, Yeganeh B et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Mijaljica D, Prescott M, Devenish RJ (2011) Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 7(7):673–682
Maycotte P, Guemez-Gamboa A, Moran J (2010) Apoptosis and autophagy in rat cerebellar granule neuron death: Role of reactive oxygen species. J Neurosci Res 88(1):73–85
Wang QJ, Ding Y, Kohtz DS et al (2006) Induction of autophagy in axonal dystrophy and degeneration. J Neurosci 26(31):8057–8068
Ma S, Attarwala IY, Xie X-Q (2019) SQSTM1/p62: A Potential Target for Neurodegenerative Disease. ACS Chem Neurosci 10(5):2094–2114
Tang SQ, Jiang QY, Yang CF et al (2010) Research and development of Lipin family. Yi Chuan 32(10):981–993
Mul JD, Nadra K, Jagalur NB et al (2011) A hypomorphic mutation in Lpin1 induces progressively improving neuropathy and lipodystrophy in the rat. J Biol Chem 286(30):26781–26793
Pan S, Fengjie Z, Feng H et al (2020) Lipin1 mediates cognitive impairment in fld mice via PKD-ERK pathway. Biochem Biophys Res Commun 525(2):286–291
Min X, Meijian W, Wei L et al (2020) Lipin1 is involved in the pathogenesis of diabetic encephalopathy through the PKD/Limk/Cofilin signaling pathway. Oxid Med Cell Longev 16(2020):1723423
Al-Rasheed NM, Al-Rasheed NM, Bassiouni YA et al (2018) Simvastatin ameliorates diabetic nephropathy by attenuating oxidative stress and apoptosis in a rat model of streptozotocin-induced type 1 diabetes. Biomed Pharmacother 105:290–298
Furman BL (2015) Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc Pharmacol 70:5.47.1-5.47.20
Wang Xu, Wang C, Zeng J et al (2005) Gene Transfer to Dorsal Root Ganglia by Intrathecal Injection: effects on Regeneration of Peripheral Nerves. Mol Ther 12(2):314–320
Njoo C, Heinl C, Kuner R (2014) In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J Vis Exp 85:51229
Taliyan R, Sharma PL (2012) Possible mechanism of protective effect of thalidomide in STZ-induced-neuropathic pain behavior in rats. Inflammopharmacology 20(2):89–97
Kishore L, Kaur N, Singh R (2018) Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology 26(4):993–1003
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25(4):402–408
Zhuang X, Li X, Qian G et al (2015) Changes of Lipin1β Expression in Gestational Diabetes Mellitus. Clin Lab 61(3–4):307–313
Luo Q, Feng Y, Xie Y et al (2019) Nanoparticle-microRNA-146a-5p polyplexes ameliorate diabetic peripheral neuropathy by modulating inflammation and apoptosis. Nanomedicine 17:188–197
Cameron NE, Eaton SE, Cotter MA, Tesfaye S (2001) Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44(11):1973–1988
Tesfaye S, Harris ND, Wilson RM, Ward JD (1992) Exercise-induced conduction velocity increment: a marker of impaired peripheral nerve blood flow in diabetic neuropathy. Diabetologia 35(2):155–159
Oates PJ (2002) Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol 50:325–392
Wada R, Yagihashi S (2005) Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci 1043:598–604
Anand P, Terenghi G, Warner G et al (1996) The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med 2(6):703–707
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL (2012) Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 11(6):521–534
Stavoe AKH, Holzbaur ELF (2019) Autophagy in Neurons. Annu Rev Cell Dev Biol 6(35):477–500
Scrivo A, Codogno P, Bomont P (2019) Gigaxonin E3 ligase governs ATG16L1 turnover to control autophagosome production. Nat Commun 10(1):780
Donkor J, Sariahmetoglu M, Dewald J et al (2007) Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem 282:3450–3457
Han GS, Wu WI, Carman GM (2006) The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem 281:9210–9218
Peixiang Zhang M, Verity A, Reue K (2014) Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab 20(2):267–279
Qu L, Zhang H, Gu B et al (2016) Jinmaitong Alleviates the diabetic peripheral neuropathy by inducing autophagy. Chin J Integr Med 22(3):185–192
Liu Y, Chen X, Yao J, Kang J (2019) Circular RNA ACR relieves high glucose-aroused RSC96 cell apoptosis and autophagy via declining microRNA-145-3p. J Cell Biochem. https://doi.org/10.1002/jcb.29568
Wang X, Huan Y, Li C et al (2020) Diphenyl diselenide alleviates diabetic peripheral neuropathy in rats with streptozotocin-induced diabetes by modulating oxidative stress. Biochem Pharmacol 182:114221
Muscella A, Vetrugno C, Cossa LG et al (2020) TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J Neurochem 153(4):525–538
Clements MP, Byrne E, Camarillo Guerrero LF et al (2017) The Wound Microenvironment Reprograms Schwann Cells to Invasive Mesenchymal-like Cells to Drive Peripheral Nerve Regeneration. Neuron 96(1):98-114.e7
Mir SU, Schwarze SR, Jin L et al (2013) Progesterone receptor membrane component 1/Sigma-2 receptor associates with MAP1LC3B and promotes autophagy. Autophagy 9(10):1566–1578
Del Bello B, Marcolongo P, Ciarmela P et al (2019) Autophagy up-regulation by ulipristal acetate as a novel target mechanism in the treatment of uterine leiomyoma: an in vitro study. Fertil Steril 112(6):1150–1159
Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M et al (2015) Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol 210(1):153–168
Towns R, Guo C, Shangguan Y et al (2008) Type 2 diabetes with neuropathy: autoantibody stimulation of autophagy via Fas. NeuroReport 19(3):265–269
Du W, Wang N, Li F et al (2019) STAT3 phosphorylation mediates high glucose-impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. FASEB J 33(7):8008–8021
Acknowledgements
Thanks for the guidance of the teachers from the Institute of basic medicine of Shandong University and the provision of the rat sciatic nerve conduction velocity meter. We also thank the teachers of the basic laboratory of the Second Hospital of Shandong University for their guidance in the research of apoptosis.
Funding
This study was supported by the National Natural Science Foundation of China (NNSFC) (NO.81670753 and NO. 82070847) to S.C., and NNSFC (NO.81800722) to X.Z., the grants from the Key R & D programs of Shandong Province (No.2018GSF118108) to S.C., and the Hospital Youth Foundation of Qilu Hospital of Shandong University, Qingdao (QDKY2017QN12) to M.W.
Author information
Authors and Affiliations
Contributions
S.C. conceived the study. X.Z., S.Y., L.C., and S.C. designed the study. M.W., M.X., P.S, C.Z., X.H., and C.F. performed the experiments and interpreted data analyses. M.W. wrote the first version of the paper. All authors critically reviewed, revised, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants performed by any of the authors. Animal experiments were performed in accordance with the International Guiding Principles for Animal Research as stipulated by the World Health Organization were followed.
Consent for Publication
All authors agree to publish the article in this magazine and the manuscript contains no any individual person’s data in any form.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Xie, M., Yu, S. et al. Lipin1 Alleviates Autophagy Disorder in Sciatic Nerve and Improves Diabetic Peripheral Neuropathy. Mol Neurobiol 58, 6049–6061 (2021). https://doi.org/10.1007/s12035-021-02540-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02540-5