Abstract
MiR-143-3p is aberrantly expressed in patients with ischemic stroke and associated with ischemic brain injury. However, the underlying mechanisms are largely unknown. Here, we confirmed circ_0025984 and TET1 as a sponge and target of miR-143-3p, respectively, by luciferase reporter assay. In astrocytes, OGD significantly decreased circ_0025984 and TET1 levels but increased miR-143-3p levels, which was also observed in brains of mice with MCAO. Treatment with miR-143-3p inhibitor or circ_0025984 significantly decreased astrocyte apoptosis and autophagy, as well as cerebral injury and neuron loss in mice with MCAO. Notably, TET1 overexpression decreased astrocyte apoptosis and autophagy and induced promoter hypomethylation and expression of ORP150. Our results demonstrated for the first time that circ_0025984 protects astrocytes from ischemia-induced autophagy and apoptosis by targeting the miR-143-3p/TET1 pathway and might inhibit cerebral injury induced by ischemic stroke. Furthermore, our data revealed the important positive regulation of ORP150 by TET1, which could be associated with its neuroprotective role.

Model for signaling pathway of circ_0025984/miR-143-3p/TET1 inastrocytes cultured under OGD. In astrocytes, circ_0025984 acts as a sponge of miR-143-3p, which directly targets TET1 and decreases its expression (A). After translocatinginto the nucleus, TET1 binds to the promoter of ORP150, converts 5mC into 5hmC,leading to DNA demethylation and increased expression of ORP150 (B). In astrocytescultured under OGD, ER stress is induced and eventually leads to apoptosis andautophagy mediated by ATG7, which is regulated by circ_0025984 via ORP150 andGRP78 (C).








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- rt-PA:
-
Recombinant tissue plasminogen activator
- CNS:
-
Central nervous system
- circRNAs:
-
Circular RNAs
- tMCAO:
-
Transient middle cerebral artery occlusion
- MCAO:
-
Middle cerebral artery occlusion
- CCA:
-
Common carotid artery
- PFA:
-
Paraformaldehyde
- DMEM:
-
Dulbecco’s modified Eagle medium
- TET1:
-
Ten-eleven translocation methylcytosine dioxygenase 1
- NCs:
-
Negative controls
- OGD:
-
Oxygen and glucose deprivation
- TEM:
-
Transmission electron microscope
- FISH:
-
Fluorescence in situ hybridization
- ChIP:
-
Chromatin immunoprecipitation
- RIP:
-
RNA immunoprecipitation
- MSP:
-
Methylation-specific PCR
- 5mC:
-
5-Methyl-cytosine
- 5hmC:
-
5-Hydroxymethyl-cytosine
- FGF:
-
Fibroblast growth factor
- BDNF:
-
Brain-derived neurotrophic factor
- ER:
-
Endoplasmic reticulum
- HMEC-1:
-
Human microvascular endothelial cells-1
- oxLDL:
-
Oxidized low density lipoprotein
References
Awada Z, Abboud R, Nasr S (2019) Risk of serious bleeding with antiplatelet therapy for secondary prevention post ischemic stroke in Middle East population. Cureus 11(6):e4942
Asadi P, Zia Ziabari SM, Naghshe Jahan D, Jafarian Yazdi A (2019) Electrocardiogram changes as an independent predictive factor of mortality in patients with acute ischemic stroke; a cohort study. Arch Acad Emerg Med 7(1):e27
van der Worp HB, van Gijn J (2007) Clinical practice. Acute ischemic stroke. N Engl J Med 357(6):572–579
Li Y, Zhong W, Jiang Z, Tang X (2019) New progress in the approaches for blood-brain barrier protection in acute ischemic stroke. Brain Res Bull 144:46–57
Lu D, Mai HC, Liang YB, Xu BD, Xu AD, Zhang YS (2018) Beneficial role of rosuvastatin in blood-brain barrier damage following experimental ischemic stroke. Front Pharmacol 9:926
Han B, Zhang Y, Zhang Y, Bai Y, Chen X, Huang R, Wu F, Leng S et al (2018) Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy 14(7):1164–1184
Swanson RA, Ying W, Kauppinen TM (2004) Astrocyte influences on ischemic neuronal death. Curr Mol Med 4(2):193–205
Zhang Z, Chopp M (2002) Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med 12(2):62–66
Liu Z, Chopp M (2016) Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol 144:103–120
Sims NR, Yew WP (2017) Reactive astrogliosis in stroke: contributions of astrocytes to recovery of neurological function. Neurochem Int 107:88–103
Qin AP, Liu CF, Qin YY, Hong LZ, Xu M, Yang L, Liu J, Qin ZH et al (2010) Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy 6(6):738–753
Qianqian Tang SW, Qiao X, Wang F, Wang Y (2020) MiR-29 promotes ovarian carcinoma cell proliferation through the PTEN pathway. European Journal of Gynaecological Oncology 41(5):774–778
Vishnoi A, Rani S (2017) MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol 1509:1–10
Tiedt S, Prestel M, Malik R, Schieferdecker N, Duering M, Kautzky V, Stoycheva I, Böck J et al (2017) RNA-Seq identifies circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ Res 121(8):970–980
Creemers EE, Tijsen AJ, Pinto YM (2012) Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 110(3):483–495
Zampetaki A, Mayr M (2012) MicroRNAs in vascular and metabolic disease. Circ Res 110(3):508–522
Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L (2015) TGFbeta triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res 116(11):1753–1764
Wang Y, Lee CGL (2009) MicroRNA and cancer--focus on apoptosis. Journal of cellular and molecular medicine 13(1):12–23
Zhang X, Dong Y, Ti H, Zhao J, Wang Y, Li T, Zhang B (2013) Down-regulation of miR-145 and miR-143 might be associated with DNA methyltransferase 3B overexpression and worse prognosis in endometrioid carcinomas. Human pathology 44(11):2571–2580
Noguchi S, Mori T, Hoshino Y, Maruo K, Yamada N, Kitade Y, Naoe T, Akao Y (2011) MicroRNA-143 functions as a tumor suppressor in human bladder cancer T24 cells. Cancer letters 307(2):211–220
Zhang N, Su Y, Xu L (2013) Targeting PKCε by miR-143 regulates cell apoptosis in lung cancer. FEBS letters 587(22):3661–3667
Liu C, Zhang C, Yang J, Geng X, du H, Ji X, Zhao H (2017) Screening circular RNA expression patterns following focal cerebral ischemia in mice. Oncotarget 8(49):86535–86547
Duan X, Li L, Gan J, Peng C, Wang X, Chen W, Peng D (2019) Identification and functional analysis of circular RNAs induced in rats by middle cerebral artery occlusion. Gene 701:139–145
Dong Z, Deng L, Peng Q, Pan J, Wang Y (2020) CircRNA expression profiles and function prediction in peripheral blood mononuclear cells of patients with acute ischemic stroke. J Cell Physiol 235(3):2609–2618
Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J et al (2018) Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci 38(1):32–50
Bertrand L, Dygert L, and Toborek M (2017) Induction of ischemic stroke and ischemia-reperfusion in mice using the middle artery occlusion technique and visualization of infarct area. J Vis Exp 2(120):54805
Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z (2000) Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab 20(9):1311–1319
Weitzmann A, Volkmer JR, Zimmermann R (2006) The nucleotide exchange factor activity of Grp170 may explain the non-lethal phenotype of loss of Sil1 function in man and mouse. FEBS Lett 580(22):5237–5240
Durukan A, Tatlisumak T (2007) Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 87(1):179–197
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994) Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25(9):1794–1798
Becerra-Calixto A, Cardona-Gomez GP (2017) The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci 10:88
Gao J, Long L, Xu F, Feng L, Liu Y, Shi J, Gong Q (2020) Icariside II, a phosphodiesterase 5 inhibitor, attenuates cerebral ischemia/reperfusion injury by inhibiting GSK-3β-mediated activation of autophagy. Br J Pharmacol 177(6):1434–1452
Pan R, Timmins GS, Liu W, Liu KJ (2015) Autophagy mediates astrocyte death during zinc-potentiated ischemia--reperfusion injury. Biol Trace Elem Res 166(1):89–95
He Z, Yi J, Liu X, Chen J, Han S, Jin L, Chen L, Song H (2016) MiR-143-3p functions as a tumor suppressor by regulating cell proliferation, invasion and epithelial–mesenchymal transition by targeting QKI-5 in esophageal squamous cell carcinoma. Molecular Cancer 15(1):51
Jin Y-P et al (2018) miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell death & disease 9(2):1–15
Yang J, Jiang B, Hai J, Duan S, Dong X, Chen C (2019) Long noncoding RNA opa-interacting protein 5 antisense transcript 1 promotes proliferation and invasion through elevating integrin α6 expression by sponging miR-143-3p in cervical cancer. J Cell Biochem 120(1):907–916
Yang Z, Wang J, Pan Z, Zhang Y (2018) miR-143-3p regulates cell proliferation and apoptosis by targeting IGF1R and IGFBP5 and regulating the Ras/p38 MAPK signaling pathway in rheumatoid arthritis. Experimental and therapeutic medicine 15(4):3781–3790
Wu X, Zhang Y (2017) TET-mediated active DNA demethylation: mechanism, function and beyond. Nature Reviews Genetics 18(9):517–534
Kim BJ, Kim Y, Hong EP, Jeon JP, Yang JS, Choi HJ, Kang SH, Cho YJ (2019) Correlation between altered DNA methylation of intergenic regions of ITPR3 and development of delayed cerebral ischemia in subarachnoid hemorrhage patients. World Neurosurg 130:e449–e456
Münzel M et al (2010) Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angewandte Chemie (International ed. in English) 49(31):5375–5377
Ruzov A, Tsenkina Y, Serio A, Dudnakova T, Fletcher J, Bai Y, Chebotareva T, Pells S et al (2011) Lineage-specific distribution of high levels of genomic 5-hydroxymethylcytosine in mammalian development. Cell research 21(9):1332–1342
Guo JU, Su Y, Zhong C, Ming GL, Song H (2011) Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145(3):423–434
Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, Kou X, Zhang Y et al (2013) Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell stem cell 12(4):453–469
Takeuchi S (2006) Molecular cloning, sequence, function and structural basis of human heart 150 kDa oxygen-regulated protein, an ER chaperone. Protein J. 25(7-8):517–528
Tamatani M, Matsuyama T, Yamaguchi A, Mitsuda N, Tsukamoto Y, Taniguchi M, Che YH, Ozawa K et al (2001) ORP150 protects against hypoxia/ischemia-induced neuronal death. Nature medicine 7(3):317–323
Sanson M, Ingueneau C, Vindis C, Thiers JC, Glock Y, Rousseau H, Sawa Y, Bando Y, Mallat Z, Salvayre R, Nègre-Salvayre A (2008) Oxygen-regulated protein-150 prevents calcium homeostasis deregulation and apoptosis induced by oxidized LDL in vascular cells. Cell Death Differ 15(8):1255–1265
Zhao L, Rosales C, Seburn K, Ron D, Ackerman SL (2010) Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of Marinesco-Sjogren syndrome. Human molecular genetics 19(1):25–35
Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12(1):105–118
Pagare PP, Wang H, Wang XY, Zhang Y (2018) Understanding the role of glucose regulated protein 170 (GRP170) as a nucleotide exchange factor through molecular simulations. J. Mol. Graph. Model. 85:160–170
Sanson M, Augé N, Vindis Ć, Muller C, Bando Y, Thiers JC, Marachet MÀ, Zarkovic K et al (2009) Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells prevention by oxygen-regulated protein 150 expression. Circulation Research 104(3):328–336
Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B (2014) Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int 68:18–27
Wang Y, Wu H, Li Z, Yang P, Li Z (2017) A positive feedback loop between GRP78 and VPS34 is critical for GRP78-mediated autophagy in cancer cells. Exp Cell Res 351(1):24–35
Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS (2008) The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ 15(9):1460–1471
Tanida I, Yamasaki M, Komatsu M, Ueno T (2012) The FAP motif within human ATG7, an autophagy-related E1-like enzyme, is essential for the E2-substrate reaction of LC3 lipidation. Autophagy 8(1):88–97
Kim WY, Nam SA, Choi A, Kim YM, Park SH, Kim HL, Kim H, Han KH et al (2019) Atg7-dependent canonical autophagy regulates the degradation of aquaporin 2 in prolonged hypokalemia. Sci Rep 9(1):3021
Pengyue Z, Tao G, Hongyun H, Liqiang Y, Yihao D (2017) Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed Pharmacother 90:69–76
Funding
This study was supported by the National Natural Science Foundation of China (No. 81860321).
The follow-up supplementary experiment for this work is supported by Regional common diseases and adult stem cell transformation research innovation platform, Science and Technology Department of Guizhou Province [Guizhou specific grant (2019) 4008].
Author information
Authors and Affiliations
Contributions
Daixuan Zhou, Zhi Huang, and Xiaoxi Zhu designed the experiments; Daixuan Zhou and Zhi Huang performed the experiments. All authors analyzed the data and prepared the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Guizhou Medical University.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1.
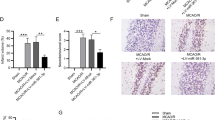
Circ_0025984 and TET1 expression in the hippocampus of the brain. Circ_0025984 level was confirmed through FISH assay in the hippocampus of the brain (A). TET1 expression was determined by IF assay in the hippocampus of the brain (B). (PNG 3349 kb)
Supplementary Figure 2.
MiR-143-3p inhibition dramatically induced neuron growth and alleviated cerebral injury in MCAO model rats. After miR-143-3p inhibition, western blot assay was adopted to evaluate c-Myc and Cyclin D1 expressions in MCAO model rats (A). And we also quantitatively analyzed the relative expressions of c-Myc and Cyclin D1 in line with the grayscale (B). After treatment with miR-143-3p inhibitor, the infarct volume was calculated through TTC staining (C). The mNSS scores were also assessed at 1, 3, 7 and 14 days in MCAO model rats (D). *p < 0.05, **p < 0.01, ***p < 0.001. (PNG 679 kb)
Supplementary Figure 3.
Circ_0025984 overexpression prominently promoted neuron growth and relieved cerebral injury in MCAO model rats. c-Myc and Cyclin D1 expressions were examined by western blot assay in MCAO model rats after circ_0025984 overexpression (A). The relative levels of c-Myc and Cyclin D1 were calculated based on the grayscale (B). We also evaluated the infarct volume (C) and mNSS scores (D) in MCAO model rats after circ_0025984 overexpression. *p < 0.05, **p < 0.01, ***p < 0.001. (PNG 682 kb)
Rights and permissions
About this article
Cite this article
Zhou, D., Huang, Z., Zhu, X. et al. Circular RNA 0025984 Ameliorates Ischemic Stroke Injury and Protects Astrocytes Through miR-143-3p/TET1/ORP150 Pathway. Mol Neurobiol 58, 5937–5953 (2021). https://doi.org/10.1007/s12035-021-02486-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02486-8