Abstract
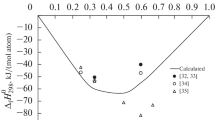
The thermodynamic characteristics of aluminum–hafnium melts are studied. The well-known (Δf\(H_{{298}}^{0}\)) thermodynamic properties are analyzed, and unknown ones ((\(S_{{298}}^{0}\)), (\(H_{{298}}^{0}\) – \(H_{0}^{0}\)), cp(T), and cp(liq)) are calculated for the congruently melting Al3Hf, Al2Hf, AlHf, and Al2Hf3 compounds. The standard formation enthalpies of these intermetallic compounds are taken from the literature, where they were calculated using semiempirical Miedema’s model and were –142.4, –134.1, –100.6, and –225 kJ/mol for each intermetallic compound, respectively. The calculation results are used for a thermodynamic simulation (TS) of Al–Hf melts. The thermodynamic simulation is performed using the TERRA software package. The composition and the thermodynamic characteristics of the melts are calculated using the model of ideal solutions of interaction products (ISIP). This model is used to study the thermodynamics of the liquid solutions in the aluminum–hafnium system. The simulation is carried out in an initial argon atmosphere at a total pressure of 105 Pa. The studies are performed in the region of temperatures and compositions corresponding to the liquid state of this system (2100–2300 K). A comparison of the results with the results of ideal solution approximation simulation makes it possible to determine the excess integral thermodynamic characteristics (enthalpy, entropy, Gibbs energy) of the Al–Hf melts. The enthalpies of mixing are shown to decrease in absolute value as temperature increases. The values found in this work are compared with the well-known data on the integral enthalpies of mixing for aluminum melts containing other III–IV group transition elements (Sc, Ti, Zr). The formation of Al–Hf, Al–Sc, Al–Ti, and Al–Zr liquid alloys is found to be accompanied by significant heat release. The extreme integral enthalpy of mixing of an Al–Hf alloy is comparable with that of an Al–Ti alloy and is no higher than –32 kJ/mol in absolute value. The components of the Al–Sc and Al–Zr systems demonstrate a tendency toward a stronger interaction: ΔHmix reaches –45 kJ/mol. The ISIP model used for TS makes it possible to describe the thermodynamic properties of the aluminum–hafnium melts.



Similar content being viewed by others
REFERENCES
N. A. Belov, A. N. Alabin, I. A. Matveeva, and D. G. Eskin, “Effect of Zr additions and annealing temperature on electrical conductivity and hardness of hot rolled Al sheets,” Trans. Nonfer. Met. Soc. China (English Edition) 25, 2817–2826 (2015).
V. V. Zakharov, “On combined alloying of aluminium alloys with scandium and zirconium,” Metalloved. Term. Obrab. Met. 708 (6), 3–8 (2014).
V. V. Zakharov, “On alloying of aluminum alloys with transition metals,” Metalloved. Term. Obrab. Met. 740 (2), 3–8 (2017).
K. E. Knipling, D. C. Dunand, and D N. Sedman, “Nucleation and precipitation strengthening in dilute Al–Ti and Al–Zr alloys,” Met. Mat. Trans. A 38, 2552–2563 (2007).
G. Ghosh and M. Asta, “First-principles calculation of structural energetic of Al–TM (TM = Ti, Zr, Hf) intermetallics,” Acta Mater. 53, 3225–3252 (2005).
E. Popova, P. Kotenkov, A. Shubin, and I. Gilev, Met. Mater. Int. (2019). https://doi.org/10.1007/S12540-019-00397-X
K. E. Knipling, D. C. Dunand, and D N. Seidman, “Precipitation evolution in Al–Zr and Al–Zr–Ti alloys during isothermal aging at 375–425°C,” Acta Mater. 56, 114–127 (2008).
G. Cacciamani, P. Riani, G. Borzone, et al., “Thermodynamic measurements and assessment of the Al–Sc system,” Intermetallics 7, 101–118 (1999).
I. N. Pyagaj and A. V. Vakhobov, “Formation enthalpy of aluminides in the Al–Sc system,” Izv. Akad. Nauk SSSR, Ser. Met., No. 5, 55–56 (1990).
V. Yu. Bodryakov, V. M. Zamyatin, O. P. Moskovskikh, et al., “Enthalpy and specific heat of multicomponent aluminum alloys in the solid and liquid states,” Rasplavy, No. 3, 3–9 (1997).
G. Ghosh, A. van de Walle, and M. Asta, “First-principles calculations of the structural and thermodynamic properties of bcc, fcc, and hcp solid solutions in the Al—TM (TM = Ti, Zr, and Hf) systems: a comparison of cluster expansion and supercell methods,” Acta. Mater. 56 (13), 3202–3221 (2008).
G. Balducci, A. Ciccioli, G. Gigli, D. Gozzi, and U. Anselmi-Tamburini, “Thermodynamic study of intermetallic phases in the Hf–Al system,” J. Alloys Compd. 220, 117–121 (1995).
J. L. Murray, A. J. McAlister, and D. J. Kahan, “The Al–Hf (aluminum–hafnium) system,” J. Phase Equilibria 19, 376–379 (1998).
T. Wang, Z. Jin, and J.-C. Zhao, Phase Equilibria 23 (5), 416–423 (2002). https://doi.org/10.1361/105497102770331361
S. V. Meschel and O. J. Kleppa, “Standards enthalpies of formation of 5d aluminides by high-temperature direct synthesis calorimetry,” J. Alloys Compd. 197 (1), 75–81 (1993).
G. K. Moiseev, N. A. Vatolin, L. A. Marshuk, and N. I. Il’inykh, Temperature Dependences of the Reduced Gibbs Energy of Some Inorganic Compounds (UrO RAN, Yekaterinburg, 1997).
G. K. Moiseev and N. A. Vatolin, Some Reguliarities of Changes and the Method of Calculation of the Thermodynamic Properties of Inorganic Compounds (UrO RAN, Yekaterinburg, 2001).
G. K. Moiseev and G. P. Vyatkin, Thermodynamic Simulation in Inorganic Systems (YuUrGU, Chelyabinsk, 1999).
A. B. Shubin, K. Yu. Shunyaev, and T. V. Kulikova, “Problem of the thermodynamic properties of liquid aluminum alloys with scandium,” Russ. Met. (Metally), No. 5, 364–369 (2008).
D. Sheng-Chao, S. Xiao, Y. Wen-Sheng, G. Han-Jie, and G. Jing, “Determination of thermodynamic properties in full composition range of Ti–Al binary melts based on atom and molecule coexistence theory,” Trans. Nonfer. Met. Soc. China 28, 1256–1264 (2018).
E. Fischer and C. Colinet, J. Phase Equilib. Diffus. 36, 404–413 (2015). https://doi.org/10.1007/S11669-015-0398-Y
ACKNOWLEDGMENTS
This work was performed on the equipment of the Collective Use Center Ural-M.
Funding
This work was supported in the framework of state assignment to the Institute of Metallurgy of Ural Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Ryzhkov
Rights and permissions
About this article
Cite this article
Gilev, I.O., Shubin, A.B. & Kotenkov, P.V. Thermodynamic Characteristics of Binary Al–Hf Melts. Russ. Metall. 2021, 919–923 (2021). https://doi.org/10.1134/S0036029521080097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521080097