Abstract
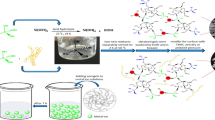
Silica aerogels have the potential use in many applications due to their large specific surface area and high porosity. One of these applications can be adsorption. However, their major disadvantage is their very low strength. Si-aerogels can be dispersed in adsorption processes with strong mechanical mixing and their removal from the solution environment becomes difficult but also their reuse becomes impossible. Therefore, mechanical strength of the adsorbent was increased with the sandwich structure synthesised in this study and it was aimed to prevent deformations during its use. In this study, a sandwich type structure with silica aerogels in the outer part and hollow silica microspheres in the inner part was synthesised. The sandwich structure was produced and characterised with a hybrid process in which production of aerogel from rice hull and hydrolysis-condensation method were used together with hollow silica microsphere production methods. The produced sandwich structure was used as an adsorbent in removing the dye material from aqueous solutions containing methylene blue (MB) dye. In the process of removing MB dye from aqueous solutions with silica aerogel-hollow silica microsphere sandwich (SAE-HS) structure, the maximum adsorption capacity was calculated as 35.94 mg/g. While it was concluded that the adsorption of MB dye with SAE-HS structure is highly dependent on pH, it was determined that there was an increase in the adsorption efficiency with the increase of pH. As a result, the sandwich structure showed a successful adsorbent property. Its high efficiency recovery was achieved. The sandwich structure has the potential to be developed as an adsorbent with a higher adsorption capacity.

Highlights
-
SAE-HS structure was successfully produced by the sol–gel method.
-
The adsorption properties of the produced structure were investigated, and the adsorption of methylene blue was successfully carried out.
-
SAE-HS structure produced and used in this study did not disperse while maintaining its strength during adsorption.
-
Modelling of adsorption of MB dye onto SAE-HS structure response surface methodology (RSM).








Similar content being viewed by others
References
Feng Q, Chen K, Ma D, Lin H, Liu Z, Qin S, Luo Y (2018) Synthesis of high specific surface area silica aerogel from rice husk ash via ambient pressure drying. Colloids Surf A 539:399–406
Hu W, Li M, Chen W, Zhang N, Li B, Wang M, Zhao Z (2016) Preparation of hydrophobic silica aerogel with kaolin dried at ambient pressure. Colloids Surf A 501:83–91
Ge D, Yang L, Li Y, Zhao J (2009) Hydrophobic and thermal insulation properties of silica aerogel/epoxy composite. J Non-Crystalline Solids 355:2610–2615
Li Z, Cheng X, Gong L, Liu Q, Li S (2018) Enhanced flame retardancy of hydrophobic silica aerogels by using sodium silicate as precursor and phosphoric acid as catalyst. J Non-Crystalline Solids 481:267–275
Mohammadian M, Kashi TSJ, Erfan M, Soorbaghi FP (2018) Synthesis and characterization of silica aerogel as a promising drug carrier system. J Drug Deliv Sci Technol 44:205–212
Han H, Wei W, Jiang Z, Lu J, Zhu J, Xie J (2016) Removal of cationic dyes from aqueous solution by adsorption onto hydrophobic/hydrophilic silica aerogel. Colloids Surf A 509:539–549
Kistler SS (1931) Coherent expanded aerogels and jellies. Nature 127:741–741
Karout A, Pierre AC (2007) Silica xerogels and aerogels synthesized with ionic liquids. J Non-Crystalline Solids 353:2900–2909
Yang H, Kong X, Zhang Y, Wu C, Cao E (2011) Mechanical properties of polymer-modified silica aerogels dried under ambient pressure. J Non-Crystalline solids 357:3447–3453
Zhu J, Xie J, Lü X, Jiang D (2009) Synthesis and characterization of superhydrophobic silica and silica/titania aerogels by sol–gel method at ambient pressure. Colloids Surf A 342:97–101
Cheng Y, Xia M, Luo F, Li N, Guo C, Wei C (2016) Effect of surface modification on physical properties of silica aerogels derived from fly ash acid sludge. Colloids Surf A 490:200–206
Başgöz Ö, Güler Ö (2020) The unusually formation of porous silica nano-stalactite structure by high temperature heat treatment of SiO2 aerogel synthesized from rice hull. Ceram Int 46:370–380
Liu T, Li Y, Du Q, Sun J, Jiao Y, Yang G, Wang Z, Xia Y, Zhang W, Wang K (2012) Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf B90:197–203
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Wu Y, Zhang L, Gao C, Ma J, Ma X, Han R (2009) Adsorption of copper ions and methylene blue in a single and binary system on wheat straw. J Chem Eng Data 54:3229–3234
Senthilkumaar S, Varadarajan P, Porkodi K, Subbhuraam C (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interface Sci 284:78–82
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigments 51:25–40
Selen V, Güler Ö, Özer D, Evin E (2016) Synthesized multi-walled carbon nanotubes as a potential adsorbent for the removal of methylene blue dye: kinetics, isotherms, and thermodynamics. Desalination Water Treat 57:8826–8838
Li Y, Du Q, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91:361–368
Gürses A, Doğar Ç, Yalçın M, Açıkyıldız M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 131:217–228
Al-Ghouti M, Khraisheh M, Allen S, Ahmad M (2003) The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J Environ Manag 69:229–238
Doğan M, Alkan M, Onganer Y (2000) Adsorption of methylene blue from aqueous solution onto perlite. Water Air Soil Pollut 120:229–248
McKay G, Porter J, Prasad G (1999) The removal of dye colours from aqueous solutions by adsorption on low-cost materials. Water Air Soil Pollut 114:423–438
Hameed B, Mahmoud D, Ahmad A (2008) Sorption equilibrium and kinetics of basic dye from aqueous solution using banana stalk waste. J Hazard Mater 158:499–506
Hameed B, Mahmoud D, Ahmad A (2008) Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J Hazard Mater 158:65–72
Hameed B (2009) Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J Hazard Mater 162:939–944
Ofomaja AE (2007) Sorption dynamics and isotherm studies of methylene blue uptake on to palm kernel fibre. Chem Eng J 126:35–43
Gong R, Li M, Yang C, Sun Y, Chen J (2005) Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J Hazard Mater 121:247–250
Meng S, Zhang J, Xu W, Chen W, Zhu L, Zhou Z, Zhu M (2019) Structural control of silica aerogel fibers for methylene blue removal. Sci China Technol Sci 62:958–964
Meng S, Zhang J, Chen W, Wang X, Zhu M (2019) Construction of continuous hollow silica aerogel fibers with hierarchical pores and excellent adsorption performance. Microporous Mesoporous Mater 273:294–296
Guzel Kaya G, Yilmaz E, Deveci H (2019) A novel silica xerogel synthesized from volcanic tuff as an adsorbent for high‐efficient removal of methylene blue: parameter optimization using Taguchi experimental design. J Chem Technol Biotechnol 94:2729–2737
Guo S, Xu H, Zhang F, Zhu X, Li X (2018) Preparation and adsorption properties of nano magnetite silica gel for methylene blue from aqueous solution. Colloids Surf A 546:244–253
Dehghani MH, Faraji M, Mohammadi A, Kamani H (2017) Optimization of fluoride adsorption onto natural and modified pumice using response surface methodology: Isotherm, kinetic and thermodynamic studies. Korean J Chem Eng 34:454–462
Ghorbani F, Kamari S (2017) Application of response surface methodology for optimization of methyl orange adsorption by Fe-grafting sugar beet bagasse. Adsorpt Sci Technol 35:317–338
Tanyildizi MS (2011) Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem Eng J 168:1234–1240
Tabassi D, Harbi S, Louati I, Hamrouni B (2017) Response surface methodology for optimization of phenol adsorption by activated carbon: Isotherm and kinetics study. Indian J Chem Techn 24:239–255
Pavlovic MD, Buntic AV, Mihajlovski KR, Siler-Marinkovic SS, Antonovic DG, Radovanovic Z, Dimitrijevic-Brankovic SI (2014) Rapid cationic dye adsorption on polyphenol-extracted coffee grounds-A response surface methodology approach. J Taiwan Inst Chem E 45:1691–1699
Vyavahare GD, Gurav RG, Jadhav PP, Patil RR, Aware CB, Jadhav JP (2018) Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 194:306–315
Khamparia S, Jaspal D (2017) Study of decolorisation of binary dye mixture by response surface methodology. J Environ Manag 201:316–326
Hong GB, Yang JX (2017) Dye removal using the solid residues from Glossogyne tenuifolia based on response surface methodology. J Mol Liq 242:82–90
Wahab MA, Jellali S, Jedidi N (2010) Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour Technol 101:5070–5075
Gao JF, Zhang Q, Su K, Chen RN, Peng YZ (2010) Biosorption of Acid Yellow 17 from aqueous solution by non-living aerobic granular sludge. J Hazard Mater 174:215–225
Youcef LD, Belaroui LS, Lopez-Galindo A (2019) Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl Clay Sci 179:105145.
Mousavi SJ, Parvini M, Ghorbani M (2018) Adsorption of heavy metals (Cu2+ and Zn2+) on novel bifunctional ordered mesoporous silica: optimization by response surface methodology. J Taiwan Inst Chem E 84:123–141
Bao Y, Wang T, Kang Q, Shi C, Ma J (2017) Micelle-template synthesis of hollow silica spheres for improving water vapor permeability of waterborne polyurethane membrane. Sci Rep 7:46638
Brinker CJ, Scherer GW. Sol-gel science: the physics and chemistry of sol-gel processing (2013). Academic Press, San Diego
ALOthman ZA (2012) A review: fundamental aspects of silicate mesoporous materials. Materials 5:2874–2902
Kivanc MR, Yonten V (2020) A statistical optimization of methylene blue removal from aqueous solutions by Agaricus Campestris using multi-step experimental design with response surface methodology: Isotherm, kinetic and thermodynamic studies. Surf Interfaces 18:100414.
Yildirim NC, Tanyol M, Yildirim N, Serdar O, Tatar S (2018) Biochemical responses of Gammarus pulex to malachite green solutions decolorized by Coriolus versicolor as a biosorbent under batch adsorption conditions optimized with response surface methodology. Ecotox Environ Safe 156:41–47
Sukriti SJ, Chadha AS, Pruthi V, Anand P, Bhatia J, Kaith BS (2017) Sequestration of dyes from artificially prepared textile effluent using RSM-CCD optimized hybrid backbone based adsorbent-kinetic and equilibrium studies. J Environ Manag 190:176–187
Mona S, Kaushik A, Kaushik CP (2011) Waste biomass of Nostoc linckia as adsorbent of crystal violet dye: Optimization based on statistical model. Int Biodeter Biodegr 65:513–521
Bandyopadhyay A, Choudhury C (2018) Crystal violet adsorption on industrial waste (hog fuel ash): equilibrium kinetics with process optimization by response surface modeling. Clean Technol Envir 20:291–308
Mojarrad M, Noroozi A, Zeinivand A, Kazemzadeh P (2018) Response surface methodology for optimization of simultaneous Cr (VI) and as (V) removal from contaminated water by nanofiltration process. Environ Prog Sustain 37:434–443
Ozdemir CS (2019) Equilibrium, kinetic, diffusion and thermodynamic applications for dye adsorption with pine cone. Sep Sci Technol 54:3046–3054
Meili L, Lins PV, Zanta CLPS, Soletti JI, Ribeiro LMO, Dornelas CB, Silva TL, Vieira MGA (2019) MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl Clay Sci 168:11–20
Karagoz S, Tay T, Ucar S, Erdem M (2008) Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour Technol 99:6214–6222
Ozer A, Pirincci HB (2006) The adsorption of Cd(II) ions on sulphuric acid-treated wheat bran. J Hazard Mater 137:849–855
Qu W, He DL, Huang HH, Guo YN, Tang YN, Song RJ (2020) Characterization of amino-crosslinked hypromellose and its adsorption characteristics for methyl orange from water. J Mater Sci 55:7268–7282
Zhu HY, Fu YQ, Jiang R, Jiang JH, Xiao L, Zeng GM, Zhao SL, Wang Y (2011) Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem Eng J 173:494–502
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GA (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 77:16–23
Shu JX, Wang ZH, Huang YJ, Huang N, Ren CG, Zhang W (2015) Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J Alloy Compd 633:338–346
Acknowledgements
The authors would like to acknowledge for the financial support of Mersin University Department of Scientific Research Projects (Project No. 2019- 3-TP3-3764).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Güler, Ö., Selen, V., Başgöz, Ö. et al. Adsorption properties and synthesis of silica aerogel-hollow silica microsphere hybrid (sandwich) structure. J Sol-Gel Sci Technol 100, 74–88 (2021). https://doi.org/10.1007/s10971-021-05622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05622-x