Abstract
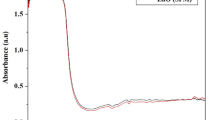
The present study deals with the synthesis of selenium nanoparticles (SeNPs) using Nilgirianthus ciliatus leaf extracts, characterized by UV–Vis spectrophotometer, XRD, FTIR, FE-SEM, HR-TEM, DLS, and zeta potential analysis. The antimicrobial activity against Staphylococcus aureus (MTCC96), Escherichia coli (MTCC443), and Salmonella typhi (MTCC98) showed the remarkable inhibitory effect at 25 µl/mL concentration level. Furthermore, the characterized SeNPs showed a great insecticidal activity against Aedes aegypti in the early larval stages with the median Lethal Concentration (LC50) of 0.92 mg/L. Histopathological observations of the SeNPs treated midgut and caeca regions of Ae. aegypti 4th instar larvae showed damaged epithelial layer and fragmented peritrophic membrane. In order to provide a mechanistic approach for further studies, molecular docking studies using Auto Dock Vina were performed with compounds of N. ciliatus within the active site of AeSCP2. Overall, the N. ciliates leaf-mediated biogenic SeNPs was promisingly evidenced to have potential larvicidal and food pathogenic bactericidal activity in an eco-friendly approach.







Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Benelli G, Mehlhorn H (2016) Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754. https://doi.org/10.1007/s00436-016-4971-z
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, MaheshKumar P, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res 114:1519–1529. https://doi.org/10.1007/s00436-015-4336-z
Karin L, Carina Z, Katja S, Adelheid O, Dominik B, Katharina B, Melanie W, Beate P, Hans PF, Franz R (2015) Mosquitoes (Diptera: Culicidae) and their relevance as disease vectors in the city of Vienna Austria. Parasitol Res 114:707–713. https://doi.org/10.1007/s00436-014-4237-6
Mehlhorn H, Al-Rasheid KA, Al-Quraishy S, Abdel-Ghaffar F (2012) Research and increase of expertise in arachno-entomology are urgently needed. Parasitol Res 110:259–265. https://doi.org/10.1007/s00436-011-2480-7
Murugan K, Panneerselvam C, Subramaniam J, Madhiyazhagan P, Hwang JS, Lan W, Dinesh D, Suresh U, Roni M, Higuchi A, Nicoletti M, Benelli G (2016) Eco-friendly drugs from the marine environment: sponge weed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ Sci Pollut Res 23:16671–16685. https://doi.org/10.1007/s11356-016-6832-9
WHO, 2017, Dengue and dengue haemorrhagic fever - fact sheet no 117, Available at, http://www.who.int/mediacentre/factsheets/fs117/en/.2017. Accessed 7 Jan
Tome HV, Pascini TV, Dangelo RA, Guedes RN, Martins GF (2014) Survival and swimming behavior of insecticide-exposed larvae and pupae of the yellow fever mosquito Aedesaegypti. Parasit Vectors 7:1–9. https://doi.org/10.1186/1756-3305-7-195
P.V. Gonzalez, L. Harburguer, P.A. Gonzalez-Audino, H.M. Masuh, The use of Aedesaegypti larvae attractants to enhance the effectiveness of larvicides, Parasitol Res 115 (2016) 2185–2190. https://doi.org/10.1007/s00436-016-4960-2
B. Morejon, F. Pilaquinga, F. Domenech, D. Ganchala, A. Debut, M. Neira (2018) Larvicidal activity of silver nanoparticles synthesized using extracts of Ambrosia arborescens (Asteraceae) to Control AedesaegyptiL. (Diptera: Culicidae), J Nanotechnol 2018. https://doi.org/10.1155/2018/6917938
P.B. Patil, K.J.Gorman, S.K. Dasgupta, K.V. Seshu Reddy, S.R. Barwale U.B. Zehr (2018) Self-limiting OX513A Aedesaegyptidemonstrate full susceptibility to currently used insecticidal chemistries as compared to Indian wild-type Aedesaegypti, Hindawi Psyche 2018. https://doi.org/10.1155/2018/781464
Govindarajan M, Benelli G (2016) α-Humulene and β-elemene from Syzygiumzeylanicum(Myrtaceae) essential oil: highly effective and eco-friendly larvicidesagainsAnophelessubpictus, Aedesalbopictus, and Culextritaeniorhynchus(Diptera:Culicidae). Parasitol Res 115:2771–2778. https://doi.org/10.1007/s00436-016-5025-2
Mishra R, Bohra A, Kamaal N, Kumar K, Gandhi K, Sujayan GK, Saabale PR, SatheeshNaik SJ, Sarma BK, Dharmendra K, Mishra M, Srivastava DK, Narendra PS (2018) Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egypt J Biol Pest Co 28:1–11. https://doi.org/10.1186/s41938-017-0004-1
Soni N, Prakash S (2014) Silver nanoparticles: a possibility for malarial and filarial vector control technology. Parasito Res 113(11):4015–4022. https://doi.org/10.1007/s00436-014-4069-4
Amita H, Snehali D, Naba Kumar M (2016) Mosquito larvicidal activity of cadmium nanoparticles synthesized from petal extracts of marigold (Tagetes sp.) and rose (Rosa sp.) flower. J Parasit. 40(4):1519–1527. https://doi.org/10.1007/s12639-015-0719-4
M. Yazhiniprabha, B. Vaseeharan, In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mater Sci Eng C 109763 (2019). https://doi.org/10.1016/j.msec.2019.109763.
Li T, Lan Q, Liu N (2009) Larvicidal activity of mosquito sterol carrier protein-2 inhibitors to the insecticide-resistant mosquito Culexquinquefasciatus(Diptera: Culicidae). J Med Entomol 46:1430–1435. https://doi.org/10.1603/033.046.0626
H. Ma, Y. Ma, X. Liu, D. H. Dyer, P. Xu, K. Liu, Q. Lan, H. Hong (2015) JianxinPeng R. Peng, NMR structure and function of Helicoverpaarmigerasterol carrier protein-2, an important insecticidal target from the cotton bollworm. Sci Rep 5:1–14. https://doi.org/10.1038/srep18186.
Dyer DH, Vyazunova I, Lorch JM, Forest KT, Lan Q (2009) Characterization of the yellow fever mosquito sterol carrier protein-2 like 3 gene and ligand-bound protein structure. Mol Cell Biochem 326:67–77. https://doi.org/10.1007/s11010-008-0007-z
Radek JT, Dyer DH, Lan Q (2010) Effects of mutations in Aedesaegyptisterol carrier protein-2 on the biological function of the protein. Biochemistry 49:7532–7541. https://doi.org/10.1021/bi902026v
Kim MS, Wessely V, Lan Q (2005) Identification of mosquito sterol carrier protein-2 inhibitors. J Lipid Res 46:650–657. https://doi.org/10.1194/jlr.M400389-JLR200
Kumar RB, Shanmugapriya B, Thiyagesan K, Kumar SR, Xavier SM (2010) A search for mosquito larvicidal compounds by blocking the sterol carrying protein, AeSCP-2, through computational screening and docking strategies. Pharmacognosy Res 2:247–253. https://doi.org/10.4103/0974-8490.69126
Singarapu KK, Ahuja A, Potula PR, Ummanni R (2016) Solution nuclear magnetic resonance studies of sterol carrier protein 2 like 2 (SCP2L2) reveal the insecticide specific structural characteristics of SCP2 proteins in Aedesaegyptimosquitoes. Biochemistry 55:4919–4927. https://doi.org/10.1021/acs.biochem.6b00322
Bintsis T (2017) Foodborne pathogens. AIMS Microbiol 3:529–563. https://doi.org/10.3934/microbiol.2017.3.529
Yang S, Xiuguo H, Ying Y, Han Q, Zhifeng Y, Yang L, Jing W, Tingting T (2018) Bacteria-Targeting nanoparticles with microenvironment-responsive antibiotic release to eliminate intracellular Staphylococcus aureus and associated infection. ACS Appl Mater Interfaces 10:14299–14311. https://doi.org/10.1021/acsami.7b15678
Joseph F, Thomas R, Webster J (2012) Cytotoxicity of selenium nanoparticles in rat dermal fibroblasts. Int J Nanomedicine 7:3907–3914. https://doi.org/10.2147/IJN.S33767
WHO, 2014, Initiative to estimate the global burden of foodborne diseases: information and publications. Geneva: World Health Organ 2014.
N. Amajoud, A. Leclercq, M. Soriano, H. Bracq-Dieye, M. El Maadoudi, N.S. Senhaji (2018) Prevalence of Listeria spp. and characterization of Listeria monocytogenes isolated from food products in Tetouan Morocco. Food Control 84:436–441. https://doi.org/10.1016/j.foodcont.2017.08.023.
Tsiraki MI, Yehia HM, Elobeid T, Osaili T, Sakkas H, Savvaidis IN (2018) Viability of and Escherichia coli O157:H7 and Listeria monocytogenesin a delicatessen appetizer (yogurt-based) salad as affected by citrus extract (Citrox©) and storage. Food Microbiol 69:11–17. https://doi.org/10.1016/j.fm.2017.07.014
C. Shi, X. Zhang, X. Zhao, R. Meng, Z. Liu, X. Chen, Na, G. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Control 71, (2017) 10–16. https://doi.org/10.1016/j.foodcont. 2016.06.020
A.A. Alfuraydi, S.M. Devanesan, M. Al-Ansari, M.S. AlSalhi, A.J. Ranjitsingh, Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancerandantimicrobialactivities. J Photochem Photobiol B 192 (2019)83–89. https://doi.org/10.1016/j.jphotobiol.2019.01.011
Oktar FN, Yetmez M, Ficai D, Ficai A, Dumitru F, Pica A (2015) Molecular mechanism and targets of the antimicrobial activity of metal nanoparticles. Curr Top Med Chem 15:1583–1588. https://doi.org/10.2174/1568026615666150414141601
Iavicoli I, Leso V, Schulte PA (2016) Biomarkers of susceptibility: state of the art and implications for occupational exposure to engineered nanomaterials. Toxicol Appl Pharmacol 299:112–124. https://doi.org/10.1016/j.taap.2015.12.018
Shakibaie M, Khorramizadeh MR, Faramarzi MR (2010) Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotech Appl Bioche 56:7–15. https://doi.org/10.1042/BA20100042
Xiao N, Lou MD, Lu YT, Yang LL, Liu Q, Liu B (2017) Ginsenoside Rg5 attenuates hepatic glucagon response via suppression of succinate-associated HIF-1α induction in HFD-fedmice. Diabetologia 60:1084–1093. https://doi.org/10.1007/s00125-017-4238-y
C. Vetrivel, K. Durairaj, D. Kalaimurugan, M. Viji, E. Murugesh, L. Wen Chao, B. Balamuralikrishnan, A. Maruthupandia Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci Rep 11 (2021) 1032. https://doi.org/10.1038/s41598-020-80327-9
Meenambigai K, Kokila R, Premkumar M, Pazhanivel T, Sivashanmugan K, Nareshkumar A (2020) Leaf extract of Dillenia indica as a source of selenium nanoparticles with larvicidal and antimicrobial potential toward vector mosquitoes and pathogenic microbes. Coatings 10:626. https://doi.org/10.3390/coatings10070626
Venkatesan A, Sujatha V (2019) Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. BioNanoScience 9:105–116. https://doi.org/10.1007/s12668-018-0566-8
Subarani S, Sabhanayakam S, Kamaraj C (2013) Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi Liston and Culexquinquefasciatus Say (Diptera: Culicidae). Parasitol Res 112:487–499. https://doi.org/10.1007/s00436-012-3158-5
Rameshkumar R, Largia MJV, Satish L, Shilpha J, Ramesh M (2017) In vitro mass propagation and conservation of Nilgirianthusciliatus through nodal explants: a globally endangered, high trade medicinal plant of Western Ghats. Plant Biosys Int J Plant Biol 151:204–211. https://doi.org/10.1080/11263504.2016.1149120
George M, Joseph L, Sony S (2017) Evaluation of analgesic activity of ethanolic extract of Strobilanthes ciliates Nees. J Pharm Innov 6:326–328
Neethu V, SheronSheeba J, Jasmine TS, Divya GS (2014) Study of phytochemical and antimicrobial potential of the leaves of Nilgirianthusciliatus Linn. Int J Appl Biol Pharm 5:150–152
Reneela P, Shubashini K (2010) Triterpenoid and sterol constituents of Strobilanthes ciliatusNees. Indian J Nat Prod 6:35–38
D.J. Tan, H. Dvinge, A. Christoforou, P. Bertone, A. Martinez Arias, K.S. Lilley, Mapping organelle proteins and protein complexes in Drosophilamelanogaster. J Proteome Res 8 (2009) 2667–2678.https://doi.org/10.1021/pr800866n.
Ramasubramanian T, Ramaraju K, Pandey SK (2015) DNA barcodes of sugarcane Aleyrodids. Int Sugar J 117:206–211
Fang C, Zhong H, Lin Y, Chen B, Han M, Ren H, Lu H, Jacob M, Luber M, Xia M, Li W, Stein S, Xu X, Zhang W, Drmanac R, Wang J, Yang H, Hammarstrom L, Aleksandar D, Kostic K, Li J (2018) Assessment of the cPAS-basedBGISEQ-500platform for meta genomic sequencing. Giga Sci 7:1–8. https://doi.org/10.1093/gigascience/gix133
WHO, (2005) Guidelines for laboratory and field testing of mosquito larvicides.Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. WHO Geneva WHO/CDS/WHOPES/GCDPP/1.3. 2005.
Roberts N (1998) Holocene book reviews on second editions. The Holocene London 8:751–754. https://doi.org/10.1191/095968398669623867
Nareshkumar A, Jeyalalitha T, Murugan K, Madhiyazhagan P (2013) Bioefficacy of plant mediated gold nanoparticles and Anthocepholuscadamba on filarial vector, Culexquinquefasciatus(Insecta: Diptera: Culicidae). Parasitol Res 112:1053–1063. https://doi.org/10.1007/s00436-012-3232-z
Azmi W, Sani RK, Banerjee UC (1998) Biodegradation of triphenylmethane dyes. Enzyme Microb Technol 22:185–191. https://doi.org/10.1016/S0141-0229(97)00159-2
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Sahar F (2010) Histopathological effects of Fenugreek (Trigonellafoenumgraceum) extracts on the larvae of the mosquito Culexquinquefasciatus. J Arab Soc Med Res 5:123–130
Fallatah SA, Khater EI (2010) Potential of medicinal plants in mosquito control. J Egypt Soc Parasitol 40:1–26
Diao WR, Hu QP, Feng SS, Li WQ, Xu JG (2013) Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylumschinifolium) against selected foodborne pathogens. J Agric Food Chem. 61:6044–6049. https://doi.org/10.1021/jf4007856
Trott O, Olson AJ (2010) AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Kim S, Cheng J, Gindulyte T, Li AJQ, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE, PubChem in, (2021) new data content and improved web interfaces. Nucleic acids Res 49(2019):D1388–D1395. https://doi.org/10.1093/nar/gkaa971
T.A. Halgren, Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94, J. Comput. Chem. 17 (1996) 490–519. https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P.
Dyer DH, Lovell S, Thoden JB, Holden HM, Rayment I, Lan Q (2003) The structural determination of an insect sterol carrier protein-2 with a ligand-bound C16 fatty acid at 1.35-Å resolution. J Biol Chem 278:39085–39091. https://doi.org/10.1074/jbc.m306214200
Ramamurthy CH, Sampath KS, Arunkumar P, SureshKumar M, Sujatha V, Premkumar K, Thirunavukkarasu C (2013) Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng 36:1131–1139. https://doi.org/10.1007/s00449-012-0867-1
Srinivasan M, Padmaja B, Sudharsan N (2013) Phytochemical identification of Nilgirianthusciliatusby GC-MS analysis and its DNA protective effect in cultured lymphocytes. Asian J Biomed Pharm Sci 3:14–17
Benelli G, Iacono AL, Canale A, Mehlhorn H (2016) Mosquito vectors and the spread of cancer:an over looked connection? Parasitol Res 115:2131–2137. https://doi.org/10.1007/s00436-016-5037-y
Li B, Webster TJ (2018) Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopaedic infections. J Orthop Res 36:22–32. https://doi.org/10.1002/jor.23656
Mishra RR, Prajapati S, Das J, Dangar TK, Das N, Thatoi H (2018) Reduction of selenite to red elemental selenium by moderately halo tolerantBacillus megateriumstrains isolated from Bhitarkanika mangrove soil and characterization of reduced product. Chemosphere 84:1231–1237. https://doi.org/10.1016/j.chemosphere.2011.05.025
Yang LB, Shen YH, Xie AJ, Liang JJ, Zhang BC (2008) Synthesis of Se nanoparticles by using TSA ion and its photocatalytic application for decolorization of congo red under UV irradiation. Mater Res Bull 43:572–582. https://doi.org/10.1016/j.materresbull.2007.04.012
Anu K, Singaravelu G, Murugan K, Benelli G (2017) Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): biophysical characterization and cytotoxicity on vero cells. J Clust Sci 28:551–563. https://doi.org/10.1007/s10876-016-1123-7
Kokila K, Elavarasan N, Sujatha V (2017) Diospyrosmontana leaf extract-mediated synthesis of selenium nanoparticles and its biological applications. New J Chem 41:7481–7490. https://doi.org/10.1039/C7NJ01124E
Gunti L, Dass RS, Kalagatur NK (2019) Phytofabrication of selenium nanoparticles from Emblicaofficinalis fruit extract and exploring its biopotential applications: antioxidant, antimicrobial, and biocompatibility. Front Microbiol 10:1–17. https://doi.org/10.3389/fmicb.2019.00931
Movasaghi Z, Rehman DI (2008) Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev 43:134–179. https://doi.org/10.1080/05704920701829043
J. Vyas S. Rana, Synthesis of selenium nanoparticles using Allium sativum extract and analysis of their antimicrobial property against gram positive bacteria. J Pharm Innov 7 (2018) 262–266.https://doi.org/10.21276/ijpbs.2019.9.1.46.
B. Fardsadegh, H. Jafarizadeh- Malmiri, Aloe vera leaf extract mediated greensynthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains, Green Process. Synth.8 (2019) 399–407. https://doi.org/10.1515/gps-2019-0007.
Mellinas C, Jimenez A, Maria D, Garrigos C (2019) Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L bean shell extract. Molecules 24:40–48. https://doi.org/10.3390/molecules24224048
Anushia C, Sampathkumar P, Ramkumar L (2009) Antibacterial and antioxidant activities in Cassia auriculata. Glob J Pharmacol 3:127–130
Vennila K, Chitra L, Balagurunathan R, Palvannan T (2018) Comparison of biological activities of selenium and silver nanoparticles attached with bioactive phytoconstituents: green synthesized using Spermacoce hispida extract. Adv Nat Sci Nanosci Nanotechnol 9:015005. https://doi.org/10.1088/2043-6254/aa9f4d
P. Sribenjarat, N. Jirakanjanakit, K. Jirasripongpun, Selenium nanoparticles biosynthesized by garlic extract as antimicrobial agent. Sci Eng Health Stud 14 (2020) 22–31. https://doi.org/10.14456/sehs.2020.3
R.S.R. Radhika, S. Gayathri, Extracelluar biosynthesis of selenium nanoparticles using some species of Lactobacillus. Indian J Geo-Mar Sci 44 (2015) 766–775. http:// hdl.handle.net/123456789/34805.
Beheshti N, Soflaei S, Shakibaie M, Yazdi MH, Ghaffarifar F, Dalimi A (2013) Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J Trace Elem Med Biol 27:203–207. https://doi.org/10.1016/j.jtemb.2012.11.002
M. Shakibaie, A.R. Shahverdi, M.A. Faramarzi, G.R. Hassanzadeh, H.R. Rahimi, O. Sabzevari, Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm Biol 51 (2013) 58–63.https://doi.org/10.3109/13880209.2012.710241
Muthukumaran U, Govindarajan M, Rajeswary M (2015) Green synthesis of silver nanoparticles from CassiaroxBurghii: a most potent power for mosquito control. Parasitol Res 114:4385–4395. https://doi.org/10.1007/s00436-015-4677-7
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2004) Biological synthesis of triangular gold nanoprisms. Nature Mater 3:482–488. https://doi.org/10.1038/nmat1152
Sowndarya P, Ramkumar G, Shivakumar MS (2017) Green synthesis of selenium nanoparticles conjugated Clausenadentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif Cells Nanomed Biotechnol. 45:1490–1495. https://doi.org/10.1080/21691401.2016.1252383
Saura SC, Hayes AW (2017) Toxicity of nanomaterials found in human environment: a literature review. Toxicol Res Appl 1:20. https://doi.org/10.1177/2397847317726352
Krebs KC, Lan Q (2003) Isolation and expression of a sterol carrier protein-2 gene from the yellow fever mosquito, Aedesaegypti. Insect Mol Biol. 12:51–60. https://doi.org/10.1046/j.1365-2583.2003.00386.x
Lan Q, Massey RJ (2004) Subcellular localization of mosquito sterol carrier protein-2 and sterol carrier protein-x. J Lipid Res 45:1468–1474. https://doi.org/10.1194/jlr.M400003-JLR200
Lan Q, Wessely V (2004) Expression of a sterol carrier protein-x gene in the yellow fever mosquito. Aedesaegypti. Insect. Mol Biol 13:519–529. https://doi.org/10.1111/j.0962-1075.2004.00510.x
Vyazunova I, Wessley V, Kim M, Lan Q (2007) Identification of two sterol carrier protein-2 like genes in the yellow fever mosquito, Aedesaegypti. Insect Mol Biol 16:305–314. https://doi.org/10.1111/j.1365-2583.2007.00729.x
Hariharan H, Al-Dhabi NA, Karuppiah P, Rajaram SK (2012) Microbial synthesis of selenium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett 9:509–515
F.J. Ramos, S.C. Chen, M.G. Garelick, D.F. Dai, C.Y. Liao, K.H. Schreiber, V.L. MacKay, E.H. An, R. Strong, W.C. Ladiges, P.S. Rabinovitch, Rapamycin reverses elevated mTORC1 signaling in lamin A/C–deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 4(2012)144ra103–144ra103. https://doi.org/10.1126/scitranslmed.3003802
Chudobova D, Simona C, Ruttkay B, Angel M, Katerina R, Kopel P, Jiri N, Sona G, Kynicky J, Vojtech K (2014) Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. FEMS Microbiol Lett 35:195–201. https://doi.org/10.1111/1574-6968.12353
Nakayama S, Ojanguren AF, Fuiman LA (2011) Process-based approach reveals directional effects of environmental factors on movement between habitats. J Anim Ecol 80:1299–1304. https://doi.org/10.1111/j.1365-2656.2011.01859.x
Carson L, Bandara S, Joseph M, Green T, Grady T, Osuji G, Weerasooriya A, Ampim P, Woldesenbet S (2020) Green synthesis of silver nanoparticles with antimicrobial properties using Phyladulcis plant extract. Foodborne Pathog Dis 17:504–511. https://doi.org/10.1089/fpd.2019.2714
Khiralla GM, El-Deeb BA (2015) Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT Food Sci Tech 63:1001–1007. https://doi.org/10.1016/j.lwt.2015.03.086
Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S (2017) Photocatalytic and antibacterial activities of hydrothermally prepared CdO nanoparticles. J Mater Sci: Mater Electron 28:11420–11429. https://doi.org/10.1007/s10854-017-6937-z
Hosseinkhani PZ, Imani AM, Rezayi S, RezaeiZarchi MS (2011) Determining the antibacterial effect of ZnO nanoparticle against the pathogenic bacterium higelladysenteriae(type1). Int J Nano Dim 1:279–285. https://doi.org/10.7508/IJND.2010.04.00
Acknowledgements
The authors sincerely acknowledge and thank Periyar University, Salem, Tamil Nadu, for providing all required laboratory facilities to conduct this research work.
Funding
Authors are thankful to the University Grants Commission (UGC), New Delhi, India, for the award of Rajiv Gandhi National Fellowship with financial support (Ref No: F1-17.1/ 2015–16/ RGNF2015-17 –SC-TAM-10313) to carry out this research work.
Author information
Authors and Affiliations
Contributions
Krishnan Meenambigai: data curation, methodology, writing – original draft; Ranganathan Kokila: methodology, formal analysis; Kandasamy Chandhirasekar: data curation, formal analysis; Ayyavu Thendralmanikandan: methodology; Durairaj Kaliannan: data curation, formal analysis; Kalibulla Syed Ibrahim: formal analysis, resources, bioinformatics; Shobana Kumar: data curation, formal analysis; Wenchao Liu: data curation, validation, writing – review and editing; Balamuralikrishnan Balasubramanian: conceptualization, writing – review and editing; Arjunan Nareshkumar: conceptualization, supervision, validation, visualization, writing – review and editing.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Balamuralikrishnan Balasubramanian is equally considered as first author.
Rights and permissions
About this article
Cite this article
Meenambigai, K., Kokila, R., Chandhirasekar, K. et al. Green Synthesis of Selenium Nanoparticles Mediated by Nilgirianthus ciliates Leaf Extracts for Antimicrobial Activity on Foodborne Pathogenic Microbes and Pesticidal Activity Against Aedes aegypti with Molecular Docking. Biol Trace Elem Res 200, 2948–2962 (2022). https://doi.org/10.1007/s12011-021-02868-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02868-y