Abstract
Background
There is no large real-world data regarding efficacy and safety of immunotherapy in gastric cancer (GC). Although some tumors can grow rapidly after immunotherapy, the patient proportions and survival outcomes are unclear in GC.
Methods
A multicenter, prospective observational study was performed to evaluate clinical outcomes including survival time, safety, and tumor behavior of nivolumab treatment for patients with advanced GC. Primary endpoint was overall survival (OS), and secondary endpoints included response rate (RR), disease control rate (DCR), progression-free survival (PFS), tumor growth rate (TGR) at first evaluation, and safety.
Results
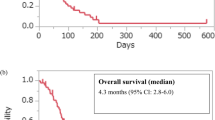
Of 501 enrolled patients, 487 were evaluable (median age 70 years, 71% male, performance status 0/1/2 [42%/44%/14%], 21% HER2-pos, 42% patients with ascites). Median OS was 5.82 months (95% CI 5.29–7.00) with a 1-year survival rate of 30% and median PFS of 1.84 months (95% CI 1.71–1.97). The DCR was 39.4% and the RR was 14.2% (95% CI 10.3–18.8) in 282 patients with measurable lesions. In 219 patients evaluable for TGR, 20.5% were identified as hyperprogressive disease (HPD). OS from the first evaluation of patients with HPD was shorter compared with non-HPD (HR 1.77, 95% CI 1.25–2.51, P = 0.001), but it was not worse than that of patients with progression and non-HPD (HR 1.05, 95% CI 0.72–1.53, P = 0.8). A multivariate analysis revealed the presence of peritoneal metastasis was a prognostic factor for OS and PFS.
Conclusions
Our real-world data demonstrated the comparable survival time to a previous clinical trial and revealed the frequency and prognosis of patients with HPD in advanced GC treated with nivolumab.
Similar content being viewed by others
Introduction
Nivolumab, a fully human antibody drug that recognizes programmed cell death-1 (PD-1), has been approved in Japan as a standard treatment for non-small cell lung cancer (NSCLC), malignant melanoma, renal cell carcinoma, classical Hodgkin’s lymphoma, head and neck cancer, malignant mesothelioma, esophageal cancer, microsatellite instability-high colorectal cancer, and gastric cancer (GC). A phase III trial (ONO-4538-12/BMS-936558 [ATTRACTION-2]) to investigate the efficacy of nivolumab for GC refractory to standard chemotherapies demonstrated the survival benefit of nivolumab as a third- or later-line treatment compared with placebo [1]. However, although the response rate (RR) was 11.2% and tumor regression was noted in approximately 40% of patients treated with nivolumab, many patients did not experience tumor regression, which suggests that nivolumab has a poor effect on the prolongation of survival in some patients. The ATTRACTION-2 trial did not enroll patients with an Eastern Cooperative Oncology Group performance status (PS) of 2 or with ascites requiring treatment; therefore, the efficacy and safety of nivolumab for patients with GC, who we commonly experience in a clinical setting, are unclear. Additionally, it has been reported that tumors grow rapidly after treatment with immune checkpoint inhibitors in several types of cancer, which is hyperprogressive disease (HPD) [2,3,4,5,6], but the patient proportions and survival outcomes are unknown in GC. Further accumulation of detailed data is desirable, including whether the content and result of prior treatments affect the efficacy and safety of nivolumab.
Therefore, this study evaluated clinical outcomes including the survival time, safety, and tumor behavior of nivolumab treatment under a clinical environment for patients with advanced GC.
Materials and methods
Study design
The DELIVER trial (JACCRO GC-08; UMIN000030850) was a multicenter, prospective observational, and translational study to examine the efficacy and toxicity of nivolumab treatment in patients with advanced GC as well as identifying novel predictors of host-related factors for nivolumab. The institutional review board at each institution approved the study protocol, and the study was conducted in accordance with the principles of the Declaration of Helsinki, and Ethical Guidelines for Medical and Health Research Involving Human Subjects. This study comprised two parts: (1) evaluation of the clinical outcomes of nivolumab treatment; and (2) identification of novel biomarkers for nivolumab. After enrollment, patients were prospectively monitored for clinical outcomes by the termination of nivolumab treatment, and imaging was performed regularly according to physicians’ decisions. Additionally, patients’ feces and blood were prospectively collected and used to measure the gut microbiome, genetic polymorphisms, gene expressions in blood, and blood metabolome, performed by a central laboratory (DNA Chip Research Inc., Tokyo, Japan). Biomarkers for efficacy and toxicity of nivolumab will be investigated using the measurement results and clinical information [7]. All participating patients provided written informed consent before study enrolment. Data were maintained by the independent JACCRO GC-08 Data Center and were analyzed by the JACCRO Statistical Analysis Department. Data and analyses were verified and assured by all academic members of the steering committee.
Study endpoints
The primary endpoint of this study was overall survival (OS). Secondary endpoints included RR, disease control rate (DCR), progression-free survival (PFS), tumor regression rate (TRR), tumor progression rate (TPR), and safety. OS was the time from enrollment to the date of death from any cause. Patients who were alive or lost to follow-up were censored at the date of last confirmation of survival. RR was the proportion of patients whose overall response was complete response (CR) or partial response (PR) for measurable lesions. DCR was the proportion of patients whose overall response was CR, PR, or stable disease (SD). The TRR or TPR was calculated based on the difference in the sum of diameters of target lesions measured according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.1 before and after nivolumab treatment. They were also calculated based on the difference in the sum of diameters between the 2 time points before nivolumab treatment. The frequency of the worst grade in all courses was calculated for each adverse event (AE) according to the Common Terminology Criteria for Adverse Events ver. 4.0. Relative dose intensity (RDI) was defined as the ratio of the dose intensity delivered to the reference standard dose intensity for a chemotherapy regimen.
Study population
This study enrolled patients who fulfilled the following criteria: (1) patients with advanced or recurrent unresectable gastric or gastroesophageal junction cancer that was histologically confirmed to be adenocarcinoma; (2) patients who will receive nivolumab 240 mg/body biweekly; (3) patients with an Eastern Cooperative Oncology Group PS of 0 to 2; (4) patients aged 20 years or older at the time of informed consent; (5) patients who were expected to be able to submit feces and undergo blood collection before the start of treatment with nivolumab and when becoming refractory/intolerant to the treatment; and (6) patients who were fully informed regarding the content of this study and personally provided written consent to participate in the study. The ineligibility criteria were as follows: patients who had previously undergone nivolumab treatment; patients with hepatitis B surface antigen or hepatitis C virus antibody; and patients considered by the investigator or sub-investigator to be inappropriate for safely participating in this study.
Definition of hyperprogressive disease
The tumor growth rate (TGR) was defined as the percentage increase in tumor volume between 2 imaging measurements in 1 month according to previous reports [8, 9]. The TGR was calculated by the sum of the diameter of target lesions according to RECIST criteria and the time interval between the two imaging assessments to compare the growth rate before and after nivolumab treatment. In this study, the HPD was defined as a ≥ twofold increase of the TGR before and after nivolumab treatment according to a previous report [2]. Additionally, another definition of the HPD based on a linear tumor growth model was used for an exploratory analysis as previously reported [10, 11]. We defined HPD as a ≥ twofold increase of the tumor growth kinetics ratio and 50% increase of the tumor burden.
Statistical design
Efficacy and safety analysis sets consisted of patients enrolled in this study and who received at least 1 course of nivolumab treatment. OS and PFS were analyzed based on the efficacy analysis set and estimated using the Kaplan–Meier method. The median survival time (MST) and survival probability at 1 and 2 years of OS with their 95% confidence intervals (CI) were estimated. The MST of OS was compared with the null hypothesis of 4.0 months using the Brookmeyer and Crowley CI method. The RR was analyzed based on the efficacy analysis set with measurable lesions. The proportion of patients whose overall response was CR or PR and the 95% CI were calculated using binomial distribution. Based on the safety analysis set, reported AEs were aggregated by grade, and their incidence and 95% CI were calculated using binomial distribution. Prognostic variables were assessed using the multivariate Cox proportional hazard model. The association between HPD and patient characteristics was analyzed using logistic regression. The significance level was set to 0.05. Analyses were performed with R version 4.0.3 (R Core Team [2020]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
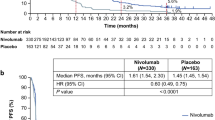
In a previous study (ATTRACTION-2 trial), the median OS was 5.3 and 4.1 months in the nivolumab and placebo groups, respectively. Based on these data, the MST of nivolumab treatment was estimated as 5 months in this study, compared with 4 months in the placebo group in the ATTRACTION-2 trial. We calculated 459 patients would have 90% power to detect a median OS difference by a two-sided test with a significance level of 0.05. Thus, the target sample size was 500 patients.
Results
Patient characteristics
Overall, 501 patients were enrolled in this study from 67 institutes between March 2018 and August 2019, and 487 patients were evaluable with clinical data. The patient cohort with measurable lesions consisted of 282 patients, and 219 patients were included in the cohort evaluable for TGR and HPD (Supplementary Fig. 1). The characteristics of 487 assessable patients are summarized in Table 1. At the time of data analysis, the median follow-up time was 5.78 (0.20–27.99) months. Sixty-seven (14%) of 487 patients had a PS of 2, and ascites and peritoneal metastases were observed in 206 (42%) and 227 (47%) patients, respectively.
Four hundred fifty-eight patients discontinued the protocol treatment and 29 patients continued to receive treatment at the data cut-off of August 2020. The median number of cycles administered per patient was 4 (1–59). At the time of this analysis, the mean RDI of nivolumab was 89.2%.
Tumor response and survival time
According to RECIST version 1.1, the CR was 1.4% and the PR was 12.8% for a RR of 14.2% (95% CI 10.3–18.8%) in patients with measurable lesions. The DCR was 39.4% (95% CI 35.1–43.9). In the survival analysis based on 389 events for OS and 454 events for PFS, the median OS was 5.82 months (95% CI 5.29–7.00) and the median PFS was 1.84 months (95% CI 1.71–1.97) (Table 2, Supplementary Fig. 2). 95% CI for median OS did not cover the Null hypothesis value of 4.0 months. Of 458 patients who discontinued treatment, the main reasons were disease progression in 393 (85.8%) and AEs in 43 (9.4%). Post-treatment after nivolumab treatment was administered to 207 of 487 patients and 191 patients received chemotherapy. Palliative radiotherapy and surgery were selected for 9 and 5 patients, respectively. In 191 patients, post-treatment regimens included irinotecan (n = 91), trifluridine/tipiracil (n = 25), irinotecan plus ramucirumab (n = 13), and fluoropyrimidine plus platinum (n = 13) (Supplementary Table 1).
Relationship between HPD and survival
In 219 patients evaluable for TGR and HPD, 62 had tumor regression and 155 had tumor progression at first evaluation. The mean TRR and TPR were 23.3% (95% CI 18.1–28.5) and 32.7% (95% CI 28.4–37.0), respectively. The tumor behavior is shown in Fig. 1. The TGR decreased after nivolumab treatment in 124 (56.6%) patients; however, 45 (20.5%) patients experienced HPD defined as a ≥ twofold increase in the TGR before and after nivolumab. A median period of 2.79 months from first evaluation to death in patients with HPD (n = 45) was shorter compared with 5.72 months in patients with non-HPD (n = 174) (HR 1.77, 95% CI 1.25–2.51, P = 0.001). The median period was comparable between the HPD and PD without HPD groups at first evaluation (n = 89) (2.79 vs. 2.40 months) (HR 1.05, 95% CI 0.72–1.53, P = 0.8) (Fig. 2, Supplementary Table 2). Patient characteristics were comparable between the HPD and non-HPD groups (Supplementary Table 3). In an exploratory analysis for the HPD based on a linear tumor growth model, 9.6% of patients were classified as HPD. We found no difference in survival time between the HPD and PD groups when using the definition of the kinetics ratio (Supplementary Table 2).
Additionally, an exploratory approach by logistic regression analysis indicated no optimal clinical factors were associated with HPD, except for the level of free-T3 in blood, which was higher in the HPD group compared with the non-HPD group (2.5 vs. 2.2 pg/ml, P = 0.005) (Table 3).
Prognostic factors in sub-group analysis by patient characteristics
Sub-group analyses of survival time, OS and PFS, by patient characteristics showed that the OS and PFS were shorter in patients with specific clinical factors (female, PS 2, albumin < 3.5, LDH ≥ 245, histology of diffuse type, peritoneal metastases, or ascites) compared with patients without (Supplementary Table 4). In the multivariate analysis, LDH ≥ 245, unresected primary tumor, and ascites were significant prognostic factors for OS, whereas male was a favorable factor for PFS. Peritoneal metastasis and PS 2 were negatively associated with OS and PFS (Table 4).
Common toxicity and immune-related toxicity
Safety was assessed in 487 patients in the safety population who received at least one course of nivolumab treatment. The overall incidences of hematological and non-hematological toxicities are shown in Table 5. Grade 3 or 4 non-hematological toxicities were anorexia (12.3%), AST increased (8.8%), fatigue (7.0%), and hyponatremia (6.0%). No treatment-related AEs leading to death were observed in this study. We performed an exploratory analysis of immune-related AEs according to the value of thyroid-related antibodies. The frequency of hyperthyroidism was 1.9% in patients with low levels of anti-thyroglobulin antibody (TgAb) (< 28 IU/mL) and 13.3% in patients with high levels of TgAb (≥ 28 IU/mL). The frequency of hyperthyroidism was 2.0% in patients with low levels of anti-thyroid peroxidase antibody (TPOAb) (< 16 IU/mL) and 8.3% in patients with high levels of TPOAb (≥ 16 IU/mL) (Supplementary Table 5).
Discussion
Our large prospective observational study demonstrated the real-world data regarding nivolumab treatment in advanced GC patients. In the ATTRACTION-2 trial, 11% of patients were previously treated with ramucirumab compared with 83% of patients in this study, which indicates that our data of nivolumab treatment for advanced GC better reflects real-world clinical practice. In our study, more patients had PS 0 and/or were treated as third-line treatment compared with the ATTRACTION-2 trial. This patients background might explain the numerically better survival time in our study. Given the good PFS results, our real-world data reconfirmed the effectiveness of nivolumab in patients with advanced GC. A sub-group analysis of this study by patient background showed that the median OS and PFS were short in patients with specific clinical factors. Patients with peritoneal dissemination had a worse OS and PFS compared with those without in multivariate analysis, which suggests that nivolumab may have a limited efficacy in GC with peritoneal dissemination. The sub-group analysis in the ATTRACTION-2 trial indicated no difference in the survival benefit of nivolumab between patients with and without peritoneal metastases [1]. However, the patient number was small in the peritoneal metastasis subgroup. A multicenter retrospective study showed that patients with peritoneal metastasis had a poor prognosis when treated with nivolumab [12]; furthermore, our study revealed the presence of peritoneal metastasis was an optimal prognostic factor in nivolumab treatment. According to our findings, patient characteristics would be clinically useful to predict the prognosis of nivolumab treatment in routine clinical practice. The prediction of nivolumab treatment outcomes by patient background may lead to treatment strategies with optimal drug selection in GC patients.
We present detailed data on HPD in nivolumab treatment for advanced GC. Although a definition of HPD has not been finalized, we utilized the definitions of two previous studies to determine HPD in this study. When we used the HPD definition that Champiat et al. defined as “tumor flares” after immunotherapy on RECIST 1.1 using the TGR before PD-1/PD-L1 treatments [2], about 20% of patients were identified as HPD, similar to previous studies of GC [11,12,13]. An analysis comparing HPD with non-HPD showed a worse prognosis in the HPD group but a comparable survival time between the HPD group and PD without HPD group. On the other hand, 9.6% of patients were classified as HPD when defined as a ≥ twofold increase of tumor growth kinetics ratio and 50% increase of tumor burden, as previously described [10]. There was no difference in survival time between the HPD and PD groups when using this definition of the kinetics ratio (Supplementary Table 2). Several studies reported no difference in survival between these two groups of GC patients [14, 15]; however, other studies showed worse survival in HPD patients compared with PD patients [12]. Our analysis evaluated the period after the determination of HPD, which would reflect the prognosis more accurately compared with the period after the initiation of nivolumab in HPD patients. In our study, the post-treatment was comparable, and there was no clear difference in patient background between the HPD and non-HPD groups (data not shown). In previous studies, HPD correlated with clinicopathological characteristics including absolute neutrophil count and C-reactive protein levels [11]; however, no correlations were observed in our study. There was a statistical association between free-T3 and HPD in the logistic regression analysis. We should further consider it because the difference in the numerical value between the groups was so small. Thus, our real-world data from a large patient cohort would provide more reliable evidence regarding the HPD rate and the prognosis compared with previous reports. Although HPD is a phenomenon representing rapid tumor growth in cancer patients, GC patients with HPD do not have worse survival compared with PD without rapid tumor growth receiving nivolumab treatment.
In this study, the toxicity profiling of immune-related AEs was similar to previous reports, and no unexpected immune-related AEs were observed. However, common AEs were observed more frequently in our study, which may reflect actual clinical practice including vulnerable patients such as those with PS 2 or ascites. Additionally, the incidence of thyroid function abnormalities induced by nivolumab was high in patients with abnormal pre-treatment thyroid function-related immune antibodies. A retrospective observational study in mainly malignant melanoma and NSCLC indicated that pre-existing TgAb were associated with thyroid dysfunction; however, the association between thyroid dysfunction and elevated TPOAb at baseline was not significant [16]. Prospective biomarker studies demonstrated the cumulative incidence of thyroid dysfunction was significantly higher in patients who were positive for anti-thyroid antibodies, TgAb and/or TPOAb, in several types of cancers including GC than those who were negative [17, 18]. Our findings support previous evidence that the clinical evaluation of anti-thyroid antibodies can predict the risk of thyroid dysfunction induced by anti-PD-1 antibodies.
In our study, images used to determine HPD were obtained by routine practice but not at protocol-specified time points. Thus, data on the RR and HPD are limited in our study. Patients defined as HPD might have included those with pseudo-progression defined as an initial increase of tumor size followed by a response to treatment, which is relatively frequent after immune-checkpoint inhibitor administration. Previously reported pseudo-progression rates were 3.7%–15.8% and 5% in melanoma and NSCLC, respectively [19,20,21]. In our study, it was difficult to discriminate between pseudo-progression and PD because of the lack of images after HPD. In the spider-plot of patients with HPD, one patient appeared to have pseudo-progression (Supplementary Fig. 3); however, a new metastatic lesion was observed in this patient when target lesions shrunk. Additionally, the current definition of HPD does not account for the occurrence of new lesions because only the sum of target lesions is used based on RECIST. There may be a difference in the impact of HPD on survival time according to the measurement method for HPD or TGR [22]. Periodic imaging studies every 2 months might lead to the accurate evaluation of HPD.
In conclusion, we obtained and analyzed large real-world data on nivolumab treatment for advanced GC to evaluate the efficacy and safety of nivolumab in a clinical environment. Our data indicated a comparable survival outcome for nivolumab treatment with a previous clinical trial. Moreover, our study revealed the rate of HPD and the prognosis of advanced GC patients treated with nivolumab. Additionally, differences in survival time by tumor response or specific clinical factors were observed and these findings might be clinically useful when considering a standard of care with nivolumab for advanced GC.
References
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71.
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–8.
Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52.
Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria J-C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748–62.
Fuentes-Antrás J, Provencio M, Díaz-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer Treat Rev. 2018;70:16–21.
Adashek JJ, Subbiah IM, Matos I, Garralda E, Menta AK, Ganeshan DM, et al. Hyperprogression and immunotherapy: fact, fiction, or alternative fact? Trends Cancer. 2020;6:181–91.
Sunakawa Y, Inoue E, Matoba R, Kawakami H, Sato Y, Nakajima TE, et al. DELIVER (JACCRO GC-08) trial: discover novel host-related immune-biomarkers for nivolumab in advanced gastric cancer. Future Oncol. 2019;15:2441–7.
Ferte C, Fernandez M, Hollebecque A, Koscielny S, Levy A, Massard C, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20:246–52.
Gomez-Roca C, Koscielny S, Ribrag V, Dromain C, Marzouk I, Bidault F, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47:2512–6.
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–50.
Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22:793–802.
Hagi T, Kurokawa Y, Kawabata R, Omori T, Matsuyama J, Fujitani K, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer. 2020;123:965–72.
Saada-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605–11.
Aoki M, Shoji H, Nagashima K, Imazeki H, Miyamoto T, Hirano H, et al. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open. 2019;4:e000488.
Suzuki H, Yamada T, Sugaya A, Ueyama S, Yamamoto Y, Moriwaki T, et al. Retrospective analysis for the efficacy and safety of nivolumab in advanced gastric cancer patients according to ascites burden. Int J Clin Oncol. 2021;26:370–7.
Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018;109:3583–90.
Okada N, Iwama S, Okuji T, Kobayashi T, Yasuda Y, Wada E, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. 2020;122:771–7.
Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc. 2018;2:241–51.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Nishino M, Giobbie-Hurder A, Manos MP, Bailey N, Buchbinder EI, Ott PA, et al. Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res. 2017;23:4671–9.
Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125–35.
Matos I, Martin-Liberal J, García-Ruiz A, Hierro C, Ochoa de Olza M, Viaplana C, et al. Capturing hyperprogressive disease with immune-checkpoint inhibitors using RECIST 11 criteria. Clin Cancer Res. 2020;26:1846–55.
Acknowledgements
We thank the patients, their families, and the investigators who participated in the DELIVER (JACCRO GC-08) trial.
Funding
This work was supported by Ono Pharmaceutical, Bristol Myers Squibb, and the Japan Clinical Cancer Research Organization (JACCRO).
Author information
Authors and Affiliations
Contributions
Conception and design: EI, HK, TEN, KM, WI, MF, YS. Collection and assembly of data: YT, EI, RK, AI, YK, YA, MT, HY, JM, AM, MT, TS, HY, HK, WI, YS. Data analysis and interpretation: EI, WI, YS. Manuscript writing: All authors Final approval manuscript: All authors Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Sunakawa reports grants and personal fees from Taiho Pharmaceutical, Chugai Pharma, Takeda, Eli Lilly Japan, and Sanofi; personal fees from Bayer Yakuhin, Yakult Honsha, Bristol-Myers Squibb Japan, Merck Biopharma, Ono Pharma, MSD, Daiichi Sankyo; and grants from Otsuka. Dr. Inoue reports personal fees from Bristol-Myers Squibb Japan, Pfizer, Nippontect systems, and RCR. Dr. Kito reports personal fees from Taiho Pharmaceutical, Chugai Pharma, Merck Biopharma, and Takeda. Dr. Takahashi reports personal fees from Takeda. Dr. Makiyama reports personal fees from Eli Lilly Japan, Taiho Pharmaceutical, Ono Pharma, Bristol-Myers Squibb Japan, and Daiichi Sankyo. Dr. Yasui reports personal fees from Daiichi Sankyo, Ono Pharma, Taiho Pharmaceutical, Bristol-Myers Squibb Japan, Chugai Pharma, Takeda, Eli Lilly Japan, TERUMO, Merck Biopharma, Yakult Honsha, and Bayer Yakuhin. Dr. Matoba reports stock of DNA chip Research Inc.. Dr. Kawakami reports grants and personal fees from Bristol-Myers Squibb Japan; personal fees from Ono Pharm. Dr. Nakajima reports grants from Sumitomo Dainippon Pharma, Boehringer Ingelheim, Bristol-Myers Squibb, Nippon Kayaku, Chugai Pharmaceutical, Sanofi, Taiho Pharmaceutical, Takeda, Eli Lilly Japan, Merck Biopharm, Daiichi Sankyo, Towa Pharmaceutical, Ono Pharm, MSD, Astellas Pharma Inc., Eisai, Solasia Pharma, Parexel International, A2 Healthecare, Mediscience Planning Inc., ICON Japan, EPS Holdings, Inc., and Japan Clinical Research Operations; personal fees from Sumitomo Dainippon Pharma, Boehringer Ingelheim, Bristol-Myers Squibb, Ono Pharm, Taiho Pharmaceutical, Takeda, Amgen Inc., Chugai Pharmaceutical, Sanofi, Novartis Japan, Nippon Kayaku, MSD, Eli Lilly Japan, Bayer Yakuhin, Pfizer, Daiichi Sankyo, Yakult Honsha, Nipro Co, Merck Biopharm, Celltrion Healthcare Japan, Teijin Pharma, and Sawai Pharmaceutical. Dr. Muro reports grants from Solasia Pharma, Merck Biopharm, Daiichi Sankyo, Parexel International, Pfizer, and MSD; grants and personal fees from Amgen, grants and personal fees from Ono Pharm, Sanofi, and Taiho Pharmaceutical; personal fees from AstraZeneca, Chugai Pharmaceutical, Takeda, Eli Lilly Japan, Bristol-Myers Squibb, and Bayer Yakuhin. Dr. Ichikawa reports grants from Takeda, Taiho Pharmaceutical, Chugai Pharmaceutical, Merck Biopharm, Shionogi, and Ono Pharmaceutical; Merck Biopharm, Chugai Pharmaceutical, and Bayer Yakuhin. All remaining authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takahashi, Y., Sunakawa, Y., Inoue, E. et al. Real-world effectiveness of nivolumab in advanced gastric cancer: the DELIVER trial (JACCRO GC-08). Gastric Cancer 25, 235–244 (2022). https://doi.org/10.1007/s10120-021-01237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01237-x