Abstract
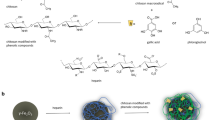
Among the available nanoparticles, those based on iron oxide are of particular interest due to their biological safety, magnetic properties, potential for imparting desired physico-chemical properties, and capacity for surface modifications with biocompatible, bioactive materials, ligands and antibodies. An important aspect of nanoparticles that determines their possibilities and range of clinical applications is their biocompatibility profile. Toxic effects can arise due to mechanisms mediated by reactive oxygen species (ROS); therefore, the development of nanoparticles intended for biomedical applications requires special attention to safety assessment in terms of the generation of oxidative stress. In this study, we examined the application of various shells (based on polylactide, polysaccharide, or albumin) on the dose-dependent effect of magnetite nanoparticles (MNPs) on the generation of ROS in stimulated human blood cells, as well as on the dynamics of the induced oxidative hemolysis of erythrocytes. MNPs with a shell of polylactide, albumin, and polysaccharide, in the range of all the used concentrations (0.1–2.0 mg/mL) and throughout the entire incubation period (0–180 min), did not affect the kinetics of the chemiluminescence response, while providing a unidirectional but differently pronounced decrease in the maximum intensity of induced chemiluminescence and total ROS production. All types of investigated nanoparticles in the range of concentrations from 1.0 to 2.0 mg/mL provided a dose-dependent enhancement of this effect. Under conditions of induced ROS generation, the various MNP shells did not modify the effects of these nanoparticles and only regulated their intensity. MNPs with a polylactide shell had a maximum effect.

Similar content being viewed by others
REFERENCES
Yaqoob AA, Ahmad H, Parveen T, Ahmad A,Oves M, Ismail IMI, Qari HA, Umar K, Mohamad Ibrahim MN (2020) Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front Chem 8:1–23. https://doi.org/10.3389/fchem.2020.00341
Filipczak N, Pan J, Yalamarty SSK, Torchilin VP (2020) Recent advancements in liposome technology. Adv Drug Deliv Rev 156:4–22. https://doi.org/10.1016/j.addr.2020.06.022
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM (2020) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res 27:19151–19168. https://doi.org/10.1007/s11356-019-05211-0
Anselmo AC, Mitragotri S (2019) Nanoparticles in the clinic: An update. Bioeng Transl Med 4:1–16. https://doi.org/10.1002/btm2.10143
Monk BJ, Herzog TJ, Wang G, Triantos S, Maul S, Knoblauch R, Mcgowan T, Shalaby WSW, Coleman RL (2019) Gynecologic Oncology A phase 3 randomized, open-label, multicenter trial for safety and ef fi cacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol Oncol 156(3):535-544 https://doi.org/10.1016/j.ygyno.2019.12.043
Srivastava V, Gusain D, Sharma YC (2015) Critical Review on the Toxicity of Some Widely Used Engineered Nanoparticles. Ind Eng Chem Res 54:6209–6233
Zhu N, Ji H, Yu P, Niu J, Farooq MU, Akram MW, Udego IO, Li H, Niu X (2018) Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 8:1–27. https://doi.org/10.3390/nano8100810
Grüttner C, Müller K, Teller J, Westphal F (2013) Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int J Hyperth 29:777–789. https://doi.org/10.3109/02656736.2013.835876
Gholami A, Mousavi SM, Hashemi SA, Chiang W, Parvin N (2020) Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab Rev 0:1–20. https://doi.org/10.1080/03602532.2020.1726943
Vallabani NVS, Singh S (2018) Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 8:1–23. https://doi.org/10.1007/s13205-018-1286-z
Kalash RS, Lakshmanan VK, Cho C-S, Park I-K (2016) Theranostics. In: Biomaterials Nanoarchitectonics. Elsevier Inc. 197–215.
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013:942916. https://doi.org/10.1155/2013/942916
Singh N, Jenkins GJS, Asadi R, Doak SH (2010) Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev 1:5358. https://doi.org/10.3402/nano.v1i0.5358
Toropova YaG, Pechnikova NA, Zelinskaya IA, Korolev DV, Gareev KG, Markitantova AS, Bogushevskaya VD, Povolotskaya AV, Manshina AA (2018) Study of hemocompatibility of magnetic magnetite nanoparticles and composite magnetite-silica particles in vitro. Bul sib med 17: 157-167. (In Russ). https://doi.org/10.20538/1682-0363-2018-3-157-167
Toropova YG, Motorina DS, Gorshkova MN, Gareev KG, Korolev DV, Muzhikyan AA (2020) The effect of intravenous administration to rats of magnetite nanoparticles with various shells on the functional state and morphology of the endothelium and on antioxidant status. Transl Med 7:52–64. https://doi.org/10.18705/2311-4495-2020-7-2-52-64
Toropova YG, Motorina DS, Zelinskaya IA, Korolev DV, Shulmeister GA, Skorik YA (2021) Generation of reactive oxygen species by human whole blood cells when exposed to iron oxide nanoparticles coated with various membranes. Bulletin of Experimental Biology and Medicine 171: 95-99. (In Russ).
DeStefano V, Khan S, Tabada A (2020) Applications of PLA in modern medicine. Eng Regen 1:76–87. https://doi.org/10.1016/j.engreg.2020.08.002
Varanko A, Saha S, Chilkoti A (2020) Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv Drug Deliv Rev 156:133–187. https://doi.org/10.1016/j.addr.2020.08.008
Wang K, Liu M, Mo R (2020) Polysaccharide-Based Biomaterials for Protein Delivery. Med Drug Discov 7:100031. https://doi.org/10.1016/j.medidd.2020.100031
Toropova YG, Golovkin AS, Malashicheva AB, Korolev DV, Gorshkov AN, Gareev KG, Afonin MV, Galagudza MM (2017) In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int J Nanomedicine 12:593–603. https://doi.org/10.2147/IJN.S122580
Gareev KG, Pugach VS, Evreinova NV, Naumysheva EB, Panov MF, Korolev DV (2017) Development of methods for surface modification of magnetic nanoparticles with a protective shell of saccharides. Biotechnosphere 3: 76-81. (In Russ).
Zorin VN, Naumisheva EB, Postnov VN, Evreinova NV, Gareev KG, Korolev DV (2018) Magnetic nanoparticles for medical application with a coating deposited with various methods. J Phys Conf Ser 1124(3): 031009. https://doi.org/10.1088/1742-6596/1124/3/031009
Dogadin SA, Dudina MA, Savchenko AA, Man’kovskii VA, Gvozdev II (2017) Respiratory burst activity in neutrophilic granulocytes in the onset of Graves’ disease. Probl Endokrinol (Mosk) 63:4–8. https://doi.org/10.14341/probl20176314-8
Dayem AA, Hossain MK, Lee S Bin, Kim K, Saha SK, Yang GM, Choi HY, Cho SG (2017) The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 18:1–21. https://doi.org/10.3390/ijms18010120
Patil US, Adireddy S, Jaiswal A, Mandava S, Lee BR, Chrisey DB (2015) In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. IntJMolSci, 16:24417–24450.
Sundarraj K, Raghunath A, Panneerselvam L, Perumal E (2017) Iron oxide nanoparticles modulate heat shock proteins and organ specific markers expression in mice male accessory organs. Toxicol Appl Pharmacol 317:12–24.
Mai T, Hilt JZ (2017) Magnetic nanoparticles: reactive oxygen species generation and potential therapeutic applications. J Nanoparticle Res 19:253. https://doi.org/10.1007/s11051-017-3943-2
Wydra RJ, Rychahou PG, Evers BM, Anderson KW, Dziubla TD, Hilt JZ (2015) The role of ROS generation from magnetic nanoparticles in an alternating magnetic field on cytotoxicity. Acta Biomater 25:284–290. https://doi.org/10.1016/j.actbio.2015.06.037
Tuzun BS, Fafal T, Tastan P, Kivcak B, Yelken BO, Kayabasi C, Susluer SY, Gunduz C (2020) Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract. Green Process Synth 9:153–163. https://doi.org/10.1515/gps-2020-0016
Paul S, Saikia JP, Samdarshi SK, Konwar BK (2009) Investigation of antioxidant property of iron oxide particlesby 1'-1' diphenylpicryl-hydrazyle (DPPH) method. J Magn Magn Mater 321:3621–3623. https://doi.org/10.1016/j.jmmm.2009.07.004
Proskurnina EV, Polimova AM, Sozarukova MM, Prudnikova MA, Ametov AS, Vladimirov YA (2016) Kinetic Chemiluminescence as a Method for Oxidative Stress Evaluation in Examinations of Patients with Type 2 Diabetes Mellitus. Bull Exp Biol Med 161:131–133. https://doi.org/10.1007/s10517-016-3362-x
Author information
Authors and Affiliations
Contributions
Idea and design of the experiment (Ya.G.T., A.Ya.B.); data collection (M.N.G., D.S.M., Yu.A.S., D.V.K., Yu.A.S., G.A.S.); data processing (Ya.G.T., M.N.G., D.S.M.); writing and editing the manuscript (Ya.G.T, E.Yu.P.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2021, Vol. 57, No. 4, pp. 310–319https://doi.org/10.31857/S0044452921040069.
Rights and permissions
About this article
Cite this article
Toropova, Y.G., Gorshkova, M.N., Motorina, D.S. et al. Influence of Iron Oxide-Based Nanoparticles with Various Shell Modifications on the Generation of Reactive Oxygen Species in Stimulated Human Blood Cells in vitro. J Evol Biochem Phys 57, 782–791 (2021). https://doi.org/10.1134/S0022093021040049
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021040049