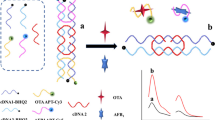
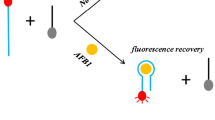
Abstract
A simple and low-cost fluorescence aptasensor was developed for rapid and sensitive signal amplification detection of T-2 mycotoxin (T-2). Dual-terminal-fluorescein amidite (FAM)–labeled aptamer (D-aptamer) acted as a recognition element and signal indicator. The metal organic frameworks (MOFs) of N, N′-bis(2-hydroxyethyl)dithiooxamidato copper (II) (H2dtoaCu) were as the quencher. The D-aptamer was initially adsorbed to the surface of H2dtoaCu, leading to efficient quenching of the aptasensor. Upon addition of T-2, the D-aptamer underwent a conformation change to form the T-2/T-2 aptamer complex, which induced the signaling probe to be released from the H2dtoaCu surface. Thus, the fluorescence intensity (FL) of the D-aptamer was recovered. Versus the single-terminal-FAM-labeled aptamer (S-aptamer), the D-aptamer showed a lower detection limit of 0.39 ng/mL. The aptasensor was also successfully applied to detect T-2 in corn and wheat samples with good recoveries.
Graphical abstract





Similar content being viewed by others
References
Li Y, Wang Z, Beier RC, Shen J, De Smet D, De Saeger S, et al. T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods. J Agric Food Chem. 2011;59:3441–53. https://doi.org/10.1021/jf200767q.
Madheswaran R, Balachandran C, Murali MB. Influence of dietary culture material containing aflatoxin and T (2) toxin on certain serum biochemical constituents in Japanese quail. Mycopathologia. 2014;158:337–41. https://doi.org/10.1007/s11046-005-8399-8.
Cho HD, Suh JH, Feng S, Eom T, Kim J, Hyun SM, et al. Comprehensive analysis of multi-class mycotoxins in twenty different species of functional and medicinal herbs using liquid chromatography–tandem mass spectrometry. Food Control. 2019;96:517–26. https://doi.org/10.1016/j.foodcont.2018.10.007.
Nualkaw K, Poapolathep S, Zhang Z, Zhang Q, Giorgi M, Li P, et al. Simultaneous determination of multiple mycotoxins in swine, poultry and dairy feeds using ultra high performance liquid chromatography-tandem mass spectrometry. Toxins. 2020;12:253–70. https://doi.org/10.3390/toxins12040253.
Juan C, Oueslati S, Mañes J, Berrada H. Multimycotoxin determination in Tunisian farm animal feed. J Food Sci. 2019;84:3885–93. https://doi.org/10.1111/1750-3841.14948.
Mahmoudi T, Miguel de la G, Behnaz S, Ahad M, Behzad B. Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. Trends Anal Chem 2019;116:13–30. https://doi.org/10.1016/j.trac.2019.04.016.
Walter H, Bauer J, Steinmeyer J, Kuzuya A, Niemeyer CM, Wagenknecht HA. “DNA origami traffic lights” with a split aptamer sensor for a bicolor fluorescence readout. Nano Lett. 2017;17:2467–72. https://doi.org/10.1021/acs.nanolett.7b00159.
Lv S, Tang Y, Zhang K, Tang D. Wet NH3-triggered NH2-MIL-125(Ti) structural switch for visible fluorescence immunoassay impregnated on paper. Anal Chem. 2018;90(24):14121–5. https://doi.org/10.1021/acs.analchem.8b04981.
Qiu Z, Shu J, Tang D. Bioresponsive release system for visual fluorescence detection of carcinoembryonic antigen from mesoporous silica nanocontainers mediated optical color on quantum dot-enzyme-impregnated paper. Anal Chem. 2017;89(9):5152–60. https://doi.org/10.1021/acs.analchem.7b00989.
Tan X, Wang X, Hao A, Liu Y, Wang X, Chu T, et al. Aptamer-based ratiometric fluorescent nanoprobe for specific and visual detection of zearalenone. Microchem J. 2020;157:104943. https://doi.org/10.1016/j.microc.2020.104943.
Pla L, Martinez-Bisbal MC, Aznar E, Sancenon F, Martinez-Manez R, Santiago-Felipe S. A fluorogenic capped mesoporous aptasensor for gluten detection. Anal Chim Acta. 2021;1147:178–86. https://doi.org/10.1016/j.aca.2020.12.060.
Zhou Q, Tang D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. Trends Anal Chem. 2020;124:115814. https://doi.org/10.1016/j.trac.2020.115814.
Lin Y, Zhou Q, Tang D, Niessner R, Knopp D. Signal-on photoelectrochemical immunoassay for aflatoxin B1 based on enzymatic product-etching MnO2 nanosheets for dissociation of carbon dots. Anal Chem. 2017;89(10):5637–45. https://doi.org/10.1021/acs.analchem.7b00942.
Lin Y, Zhou Q, Tang D. Dopamine-loaded liposomes for in-situ amplified photoelectrochemical immunoassay of AFB1 to enhance photocurrent of Mn2+-doped Zn3(OH)2V2O7 nanobelts. Anal Chem. 2017;89(21):11803–10. https://doi.org/10.1021/acs.analchem.7b03451.
Sun YF, Li S, Chen RP, Wu P, Liang J. Ultrasensitive and rapid detection of T-2 toxin using a target-responsive DNA hydrogel. Sensors Actuators B Chem. 2020;311:27912–127919. https://doi.org/10.1016/j.snb.2020.127912.
Wang B, Wu YY, Chen YF, Weng B, Xu LQ, Li CM. A highly sensitive aptasensor for OTA detection based on hybridization chain reaction and fluorescent perylene probe. Biosens Bioelectron. 2016;81:125–30. https://doi.org/10.1016/j.bios.2016.02.062.
Ji Z, Wang HZ, Canossa S, Wuttke S, Yaghi OM. Pore chemistry of metal–organic frameworks. Adv Funct Mater. 2020;30:2000238–61. https://doi.org/10.1002/adfm.202000238.
Mayer DC, Zarȩba JK, Gabriele RS, Alexander P, Marta C, Robert Z, et al. Postsynthetic framework contraction enhances the two-photon absorption properties of pillar-layered metal–organic frameworks. Chem Mater. 2020;32:5682–90. https://doi.org/10.1021/acs.chemmater.0c01417.
Dai SL, Wu SJ, Duan N, Chen J, Zheng ZG, Wang ZP. An ultrasensitive aptasensor for ochratoxin A using hexagonal core/shell upconversion nanoparticles as luminophores. Biosens Bioelectron. 2017;91:538–44. https://doi.org/10.1016/j.bios.2017.01.009.
Gao ZH, Xu BY, Zhang TJ, Liu Z, Zhang WG, Sun X, et al. Spatially responsive multicolor lanthanide-MOFs heterostructures for covert photonic barcodes. Angew Chem Int Ed. 2020;59:1–6. https://doi.org/10.1002/anie.202009295.
Khan IM, Zhao S, Niazi S, Mohsin A, Shoaib M, Duan N, et al. Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sens. Actuators, B. 2018;277:328–335.
Khan IM, Niazi S, Yu Y, Mohsin A, Mushtaq BS, Iqbal MW, et al. Aptamer induced multi-colored AuNCs-WS2 “turn on” FRET nano platform for dual-color simultaneous detection of aflatoxin B1 and zearalenone. Anal Chem. 2019;91:14085–92. https://doi.org/10.1021/acs.analchem.9b03880.
Khan R, Sherazi TA, Catanante G, Rasheed S, Marty JL, et al. Switchable fluorescence sensor toward PAT via CA-MWCNTs quenched aptamer-tagged carboxyfluorescein. Food Chem. 2020;312:126048–55. https://doi.org/10.1016/j.foodchem.2019.126048.
Li YP, Sun LL, Zhao Q. Development of aptamer fluorescent switch assay for aflatoxin B1 by using fluorescein-labeled aptamer and black hole quencher1-labeled complementary DNA. Anal Bioanal Chem. 2018;410:6269–77. https://doi.org/10.1007/s00216-018-1237-x.
Hai XM, Li N, Wang K, Zhang ZQ, Zhang J, Dang FQ. A fluorescence aptasensor based on two-dimensional sheet metal-organic frameworks for monitoring adenosine triphosphate. Anal Chim Acta. 2018;998:60–6. https://doi.org/10.1016/j.aca.2017.10.028.
Zhao XD, Wang Y, Li JZ, Huo BY, Huang H, Bai JL, et al. A fluorescence aptasensor for the sensitive detection of T-2 toxin based on FRET by adjusting the surface electric potentials of UCNPs and MIL-101. Anal Chim Acta. 2021;1160:338450–9. https://doi.org/10.1016/j.aca.2021.338450.
Zhao XD, Wang Y, Li JZ, Huo BY, Qin YK, Zhang JY, et al. A fluorescence aptasensor based on controlled zirconium–based MOFs for the highly sensitive detection of T–2 toxin. Spectrochim Acta Part A. 2021;259:119893–902. https://doi.org/10.1016/j.saa.2021.119893.
Wen XY, Huang QW, Nie DX, Zhao XY, Cao HJ, Wu WH, et al. A multifunctional N-doped Cu–MOFs (N–Cu–MOF) nanomaterial-driven electrochemical aptasensor for sensitive detection of deoxynivalenol. Molecules. 2021;26(8):2243–53. https://doi.org/10.3390/molecules26082243.
Chen XJ, Huang YK, Duan N, Wu SJ, Xia Y, Ma XY, et al. Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J Agric Food Chem. 2014;62:10368–74. https://doi.org/10.1021/jf5032058.
Zhang M, Wang Y, Yuan S, Sun X, Huo BY, Bai JL, et al. Competitive fluorometric assay for the food toxin T-2 by using DNA-modified silver nanoclusters, aptamer-modified magnetic beads, and exponential isothermal amplification. Microchim Acta. 2019;186:219–25. https://doi.org/10.1007/s00604-019-3322-z.
De Silva AP, Gunaratne HQ, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, et al. Signaling recognition events with fluorescent sensors and switches. Chem Rev. 1997;97:1515–66. https://doi.org/10.1021/cr960386p.
Li X, Zheng L, Huang L, Zheng O, Lin Z, Guo L, et al. Adsorption removal of crystal violet from aqueous solution using a metal-organic frameworks material, copper coordination polymer with dithiooxamide. J Appl Polym Sci. 2013;129:2857–64. https://doi.org/10.1002/app.39009.
He DY, Wu ZZ, Cui B, Xu EB, Jin ZY. Building a fluorescent aptasensor based on exonuclease-assisted target recycling strategy for one-step detection of T-2 toxin. Food Anal Methods. 2019;12:625–32. https://doi.org/10.1007/s12161-018-1392-x.
Zhu QY, Li TT, Ma Y, Wang ZH, Huang JX, Liu RN, et al. Colorimetric detection of cholic acid based on an aptamer adsorbed gold nanoprobe. RSC Adv. 2017;7:19250–6. https://doi.org/10.1039/c7ra00255f.
Bernhardt K, Valenta H, Kersten S, Humpf HU, Dänicke S. Determination of T-2 toxin, HT-2 toxin, and three other type A trichothecenes in layer feed by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS)-comparison of two sample preparation methods. Mycotoxin Res. 2016;32:89–97. https://doi.org/10.1007/s12550-016-0244-z.
Li YS, Chen AQ, Mao X, Sun MX, Yang SP, Li J, et al. Multiple antibodied based immunoaffinity columns preparation for the simultaneous analysis of deoxynivalenol and T-2 toxin in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2021;337:127802–7. https://doi.org/10.1016/j.foodchem.2020.127802.
Deng Q, Qiu M, Wang YL, Lv PL, Wu CJ, Sun LJ, et al. A sensitive and validated immunomagnetic-bead based enzyme-linked immunosorbent assay for analyzing total T-2 (free and modified) toxins in shrimp tissues. Ecotoxicol Environ Saf. 2017;142:441–7. https://doi.org/10.1016/j.ecoenv.2017.04.037.
Zhong HT, Yu C, Gao RF, Chen J, Yu YJ, Geng HQ, et al. A novel sandwich aptasensor for detecting T-2 toxin based on rGO-TEPA-Au@Pt nanorods with a dual signal amplification strategy. Biosens Bioelectron. 2019;144:111635–42. https://doi.org/10.1016/j.bios.2019.111635.
Guo T, Wang C, Zhou H, Zhang Y, Ma L, Wang S. A facile aptasensor based on polydopamine nanospheres for high-sensitivity sensing of T-2 toxin. Anal Methods. 2021;13(24):2654–8. https://doi.org/10.1039/d1ay00642h.
Acknowledgements
This work was supported by the Key Technologies Research and Development Program of China (2018YFC1604100), National Natural Science Foundation of China (21904064, 21878154), and Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJB150028).
Author information
Authors and Affiliations
Contributions
Xinliu Tan: methodology, investigation, roles/writing—original draft
Weidao Yu: investigation
Yuwen Wang: funding acquisition
Ping Song: supervision
Qing Xu: funding acquisition
Dengming Ming: conceptualization
Yaqiong Yang: visualization, writing—review and editing, funding acquisition
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary data associated with this article can be found in the online version at xxx.
ESM 1
(DOCX 719 kb)
Rights and permissions
About this article
Cite this article
Tan, X., Yu, W., Wang, Y. et al. A switchable and signal-amplified aptasensor based on metal organic frameworks as the quencher for turn-on detection of T-2 mycotoxin. Anal Bioanal Chem 413, 6595–6603 (2021). https://doi.org/10.1007/s00216-021-03625-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03625-9