Abstract
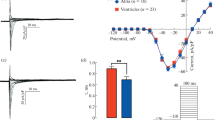
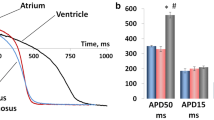
Birds acquired endothermy and a four-chambered heart independently from mammals in the course of evolution. Though avian embryos are widely used in experiments, little is known about the adult avian heart. Recent studies have shown that, despite a large evolutionary distance, the set of repolarizing potassium currents in avian myocardium resembles that in mammalian heart as well as that in humans. This allows for proposing birds as a potential model in experimental cardiology. The present study for the first time describes inward rectifier currents in working myocardium of quail. Using patch clamp method, we recorded main background inward rectifier current IK1 was recorded in isolated atrial and ventricular cardiomyocytes of quail. Both inward and outward components of IK1 in ventricular cells were larger than those in atrial cells, while there were no differences in voltage dependence of inward rectification. Acetylcholine and carbachol induced activation of acetylcholine-dependent inward rectifier current IKACh in atrial but not in ventricular myocytes. IKACh in atrial myocytes was sensitive to tertiapin. Constitutively active IKACh has not been detected. In multicellular preparations of the quail right atrium, carbachol induced hyperpolarization and shortening of action potentials, while no such effects were observed in preparations of the right ventricle. Activation of IKACh upon application of carbachol was dose-dependent with EC50 = 4.922 × 10–7 М. The described distribution of inward rectifier currents in avian myocardium is similar to that in mammalian species, which are widely used as model objects in experimental cardiology.



Similar content being viewed by others
REFERENCES
Shiels, H.A. and Galli, G.L.J., The sarcoplasmic reticulum and the evolution of the vertebrate heart, Physiology, 2014, vol. 29, no. 6, pp. 456–469.
Jensen, B., Wang, T., Christoffels, V.M., and Moorman, A.F.M., Evolution and development of the building plan of the vertebrate heart, Biochim. Biophys. Acta. Mol. Cell Res., 2013, vol. 1833, no. 4, pp. 783–794.
Filatova, T.S., Abramochkin, D.V., Pavlova, N.S., Pustovit, K.B., Konovalova, O.P., Kuzmin, V.S., and Dobrzynski, H., Repolarizing potassium currents in working myocardium of Japanese quail: Novel translational model for cardiac electrophysiology, Comp. Biochem. Physiol. Part A Mol. Integr. Physiol., 2021, vol. 255, 110919.
Filatova, T.S., Abramochkin, D.V., and Shiels, H.A., Warmer, faster, stronger: Ca2+ cycling in avian myocardium, J. Exp. Biol., 2020, vol. 223, no. 19, jev228205.
Vornanen, M., Hassinen, M., and Haverinen, J., Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current, Mar. Drugs, 2011, vol. 9, no. 11, pp. 2409–2422.
Abramochkin, D.V., Matchkov, V., and Wang, T., A characterization of the electrophysiological properties of the cardiomyocytes from ventricle, atrium and sinus venosus of the snake heart, J. Comp. Physiol. B Biochem. Syst. Environ. Physiol., 2020, vol. 190, no. 1, pp. 63–73.
Cavero, I. and Crumb, W., Native and cloned ion channels from human heart: Laboratory models for evaluating the cardiac safety of new drugs, Eur. Hear. J. Suppl., 2001, vol. 3, suppl. K, pp. K53–K63.
Jost, N., Virag, L., Bitay, M., Takács, J., Lengyel, C., Biliczki, P., Nagy, Z., Bogats, G., Lathrop, D.A., Papp, J.G., and Varró, A., Restricting excessive cardiac action potential and QT prolongation: A vital role for I Ks in human ventricular muscle, Circulation, 2005, vol. 112, no. 10, pp. 1392–1399.
Ehrlich, J.R., Inward rectifier potassium currents as a target for atrial fibrillation therapy, J. Cardiovasc. Pharmacol., 2008, vol. 52, no. 2, pp. 129–135.
Klein, M.G., Shou, M., Stohlman, J., Solhjoo, S., Haigney, M., Tidwell, R.R., Goldstein, R.E., Flagg, T.P., and Haigney, M.C., Role of suppression of the inward rectifier current in terminal action potential repolarization in the failing heart, Heart Rhythm, 2017, vol. 14, no. 8, pp. 1217–1223.
Isenberg, G. and Klockner, U., Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium,” Pflügers Arch. Eur. J. Physiol., 1982, vol. 395, no. 1, pp. 6–18.
Valance, D., Despres, G., Richard, S., Constantin, P., Mignon-Grasteau, S., Leman, S., Boissy, A., Faure, J.M., and Leterrier, C., Changes in heart rate variability during a tonic immobility test in quail, Physiol. Behav., 2008, vol. 93, no. 3, pp. 512–520.
Haverinen, J. and Vornanen, M., Responses of action potential and K+ currents to temperature acclimation in fish hearts: Phylogeny or thermal preferences?, Physiol. Biochem. Zool., 2009, vol. 82, no. 5, pp. 468–482.
Varro, A., Nanasi, P.P., and Lathrop, D.A., Potassium currents in isolated human atrial and ventricular cardiocytes, Acta Physiol. Scand., 1993, vol. 149, no. 2, pp. 133–142.
Panama, B.K., McLerie, M., and Lopatin, A.N., Heterogeneity of I K1 in the mouse heart, Am. J. Physiol. Hear. Circ. Physiol., 2007, vol. 293, no. 6, pp. H3558–H3567.
Ward, C.A., Ma, Z., Lee, S.S., and Giles, W.R., Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis, Am. J. Physiol. Gastrointest. Liver Physiol., 1997, vol. 273, no. 2, pp. G537–G544.
Hassinen, M., Haverinen, J., Hardy, M.E., Shiels, H.A., and Vornanen, M., Inward rectifier potassium current (I K1) and Kir2 composition of the zebrafish (Danio rerio) heart, Pflügers Arch. Eur. J. Physiol., 2015, vol. 467, no. 12, pp. 2437–2446.
Skarsfeldt, M.A., Bomholtz, S.H., Lundegaard, P.R., Lopez-Izquierdo, A., Tristani-Firouzi, M., and Bentzen, B.H., Atrium-specific ion channels in the zebrafish-a role of I KACh in atrial repolarization, Acta Physiol., 2018, vol. 223, no. 3, e13049.
Dobrzynski, H., Marples, D.D.R., Musa, H., Yamanu-shi, T.T., Henderson, Z., Takagishi, Y., Honjo, H., Kodama, I., and Boyett, M.R., Distribution of the muscarinic K+ channel proteins Kir3.1 and Kir3.4 in the ventricle, atrium, and sinoatrial node of heart, J. Histochem. Cytochem., 2001, vol. 49, no. 10, pp. 1221–1234.
Liang, B., Nissen, J.D., Laursen, M., Wang, X., Skibsbye, L., Hearing, M.C., Andersen, M.N., Rasmussen, H.B., Wickman, K., Grunnet, M., Olesen, S.-P., and Jespersen, T., G-protein-coupled inward rectifier potassium current contributes to ventricular repolarization, Cardiovasc. Res., 2014, vol. 101, no. 1, pp. 175–184.
Beckmann, C., Rinne, A., Littwitz, C., Mintert, E., Bosche, L.I., Kienitz, M.-C., Pott, L., and Bender, K., G protein-activated (GIRK) current in rat ventricular myocytes is masked by constitutive inward rectifier current (I K1), Cell. Physiol. Biochem., 2008, vol. 21, no. 4, pp. 259–268.
Dobrev, D., Graf, E., Wettwer, E., Himmel, H.M., Hála, O., Doerfel, C., Christ, T., Schuler, S., and Ravens, U., Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (I K,ACh) in chronic human atrial fibrillation decrease in GIRK4 mRNA correlates with reduced I K,ACh and muscarinic receptor-mediated shortening of action potentials, Circulation, 2001, vol. 104, no. 21, pp. 2551–2557.
Abramochkin, D.V. and Vornanen, M., Seasonal changes of cholinergic response in the atrium of Arctic navaga cod (Eleginus navaga), J. Comp. Physiol. B Biochem. Syst. Environ. Physiol., 2017, vol. 187, no. 2, pp. 329–338.
Lomax, A.E., Rose, R.A., and Giles, W.R., Electrophysiological evidence for a gradient of G protein-gated K+ current in adult mouse atria, Br. J. Pharmacol., 2003, vol. 140, no. 3, pp. 576–584.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 19-34-90142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interests.
Statement on the welfare of animals. All experiments were performed in accordance with Directive 86/609/EEC on the handling of laboratory animals.
Additional information
Translated by P. Kuchina
About this article
Cite this article
Filatova, T.S., Abramochkin, D.V. Inward Rectifier Currents IK1 and IKACh in Working Myocardium of Japanese Quail (Coturnix japonica). Moscow Univ. Biol.Sci. Bull. 76, 65–70 (2021). https://doi.org/10.3103/S0096392521020012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0096392521020012