Abstract
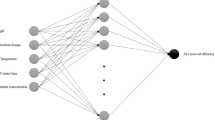
In the current study, lake sediment, a heterogeneous and complex organic matter, utilized as a catalyst upon acid treatment for efficient hydrogen generation from sodium borohydride. In order to synthesise the catalyst that bears the best catalytic activity, ANOVA, cubic stepwise linear regression and artificial neural network optimization techniques were applied to determine the optimal level of treatment parameters. The results suggest that only Taguchi orthogonal arrays method was able to accurately reflect the overall surface of objective variable. Among the 16 catalyst samples Exp(15) showed the superior catalytic activity followed by Exp(13), Exp(12), Exp(14) and Exp(7). The minimum reaction completion time for Exp(15) corresponding to maximum hydrogen production rate of 3247.15 mL/min/gcat was 2.25 min. A detailed characterization of the final product was carried out by using a Fourier transform infrared spectra (FTIR—Perkin Elmer), an X-ray diffractometer (Bruker D8 Advance XRD), a scanning electron microscopy and energy dispersive X-ray spectroscopy.
Graphic abstract








Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Midilli A, Dincer I (2008) Hydrogen as a renewable and sustainable solution in reducing global fossil fuel consumption. Int J Hydrogen Energy 33(16):4209–4222
Barbir F, Veziroǧlu T, Plass H Jr. (1990) Environmental damage due to fossil fuels use. Int J Hydrogen Energy 15(10):739–749
Panwar N, Kaushik S, Kothari S (2011) Role of renewable energy sources in environmental protection: a review. Renew Sustain Energy Rev 15(3):1513–1524
Bekirogullari M (2019) Catalytic activities of non-noble metal catalysts (CuB, FeB, and NiB) with C. Vulgaris microalgal strain support modified by using phosphoric acid for hydrogen generation from sodium borohydride methanolysis. Int J Hydrogen Energy 44(29):14981–14991
Jain I (2009) Hydrogen the fuel for 21st century. Int J Hydrogen Energy 34(17):7368–7378
Züttel A et al (1923) Hydrogen: the future energy carrier. Philos Trans Royal Soc A 2010(368):3329–3342
Biniwale RB et al (2008) Chemical hydrides: a solution to high capacity hydrogen storage and supply. Int J Hydrogen Energy 33(1):360–365
Mustafa K, Bekirogullari M (2019) Investigation of hydrogen production from sodium borohydride methanolysis in the presence of Al2O3/spirulina platensis supported Co catalyst. Eur J Lipid Sci Technol 16:69–76
Özkan G, Akkuş MS, Özkan G (2019) The effects of operating conditions on hydrogen production from sodium borohydride using Box-Wilson optimization technique. Int J Hydrogen Energy 44(20):9811–9816
Loghmani MH, Shojaei AF, Khakzad M (2017) Hydrogen generation as a clean energy through hydrolysis of sodium borohydride over Cu–Fe–B nano powders: effect of polymers and surfactants. Energy 126:830–840
Kim P et al (2006) NaBH4-assisted ethylene glycol reduction for preparation of carbon-supported Pt catalyst for methanol electro-oxidation. J Power Sources 160(2):987–990
Deraedt C et al (2014) Sodium borohydride stabilizes very active gold nanoparticle catalysts. ChemComm 50(91):14194–14196
Kaya M (2019) NiB loaded acetic acid treated microalgae strain (Spirulina platensis) to use as a catalyst for hydrogen generation from sodium borohydride methanolysis. Energy Source Part A 41(20):2549–2560
Saka C, Kaya M, Bekiroğullari M (2020) Spirulina microalgal strain as efficient a metal-free catalyst to generate hydrogen via methanolysis of sodium borohydride. Int J Energy Res 44(1):402–410
Xu D et al (2018) Efficient catalytic properties of SO42−/MxOy (M = Cu, Co, Fe) catalysts for hydrogen generation by methanolysis of sodium borohydride. Int J Hydrogen Energy 43(13):6594–6602
Lo C-TF, Karan K, Davis B (2009) Kinetic assessment of catalysts for the methanolysis of sodium borohydride for hydrogen generation. Ind Eng Chem Res 48(11):5177–5184
Saka C, Kaya M, Bekiroğullari M (2020) Spirulina Platensis microalgae strain modified with phosphoric acid as a novel support material for Co–B catalysts: its application to hydrogen production. Int J Hydrogen Energy 45(4):2872–2883
Saka C, Kaya M, Bekiroğullari M (2020) Chlorella vulgaris microalgae strain modified with zinc chloride as a new support material for hydrogen production from NaBH4 methanolysis using CuB, NiB, and FeB metal catalysts. Int J Hydrogen Energy 45(3):1959–1968
Bekirogullari M (2020) Hydrogen production from sodium borohydride by ZnCl2 treated defatted spent coffee ground catalyst. Int J Hydrogen Energy 45(16):9733–9743
El-Moslamy SH et al (2017) Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA. F4 and its application against phytopathogens. Sci Rep 7(1):1–22
Alam H, Moghaddam A, Omidkhah M (2009) The influence of process parameters on desulfurization of Mezino coal by HNO3/HCl leaching. Fuel Process Technol 90(1):1–7
Das SK, Sahoo P (2011) Tribological characteristics of electroless Ni–B coating and optimization of coating parameters using Taguchi based grey relational analysis. Mater Des 32(4):2228–2238
Singh JP, Kumar S, Mohapatra S (2018) Chemical treatment of low-grade coal using Taguchi approach. Part Sci Technol 38:73–80
Sağır K, Elçiçek H, Özdemir OK (2021) Optimization of catalyst preparation conditions for hydrogen generation in the presence of Co–B using Taguchi method. Int J Hydrogen Energy 46(7):5689–5698
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8(5):551–561
Desai KM et al (2008) Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: case study of fermentative production of scleroglucan. Biochem Eng J 41(3):266–273
Dauvalter V et al (2011) Chemical composition of lake sediments along a pollution gradient in a Subarctic watercourse. J Environ Sci Health A 46(9):1020–1033
Nriagu J, Dell C (1974) Diagenetic formation of iron phosphates in recent lake sediments. Am Mineral 59(9–10):934–946
LeNail A (2019) Nn-svg: publication-ready neural network architecture schematics. J Open Source Softw 4(33):747
Gnanasaravanan S, Rajkumar P (2013) Characterization of minerals in natural and manufactured sand in Cauvery River belt, Tamilnadu, India. Infrared Phys Technol 58:21–31
Dupuis T et al (1984) Etude par spectrographie Infrarouge d’un encroutement calcaire sous galet. Mise en évidence et modélisation expérimentale d’une suite minérale évolutive à partir de carbonate de calcium amorphe. Clay Miner 19(4):605–614
Laves F (1952) Phase relations of the alkali feldspars: I. Introductory remarks. J Geol 60(5):436–450
Tyagi B, Chudasama CD, Jasra RV (2006) Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 64(2):273–278
Gao Y, Han Y, Li W (2018) Flotation behavior of diatomite and albite using dodecylamine as a collector. Minerals 8(9):371
Milinovic J et al (2020) XRD identification of ore minerals during cruises: refinement of extraction procedure with sodium acetate buffer. Minerals 10(2):160
Sánchez-Soto PJ et al (2021) Mining wastes of an albite deposit as raw materials for vitrified mullite ceramics. Minerals 11(3):232
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bekirogullari, M., Abut, S., Duman, F. et al. Lake sediment based catalyst for hydrogen generation via methanolysis of sodium borohydride: an optimization study with artificial neural network modelling. Reac Kinet Mech Cat 134, 57–74 (2021). https://doi.org/10.1007/s11144-021-02057-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02057-x