Abstract
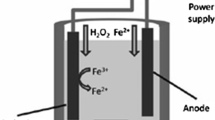
In this study, the treatment of nanofiltration (NF) concentrate from a landfill leachate treatment plant through the electrooxidation (EO) process was investigated. Experimental studies were conducted in a batch reactor equipped with multi-metal oxide/TiO2 doped Ti anode and Pt doped Ti cathode. Persulfate was added as the oxidant and the performance of Fe0, Fe2+, and Fe3+ species as catalysts for persulfate activation was compared. Kinetic studies were carried out for chemical oxygen demand (COD) removal from leachate NF concentrate. Maximum COD removal efficiency was obtained by the EO process in which persulfate was added as the oxidant and Fe2+ was used as the catalyst for persulfate activation. Optimum values of the process variables (pH 5, current 2 A, Fe2+ dose 12 mM and persulfate dose: 60 mM) were determined. The COD removal was 77.1% under optimum conditions. The overall COD removal mechanism is well fitted with the pseudo-first-order and second-order kinetic models with R2 values of > 0.80 under all applied current values. Specific energy consumption calculated for optimum conditions were 73.4 kWh/m3 and 14.3 kWh/kg COD. The EO process assisted with Fe2+ activated persulfate can be evaluated as an efficient treatment method for leachate NF concentrate treatment.
Article Highlights
-
SO 4 − . was produced by electrochemically activation.
-
Fe 0 , Fe 2+ , and Fe 3+ species as catalysts were compared.
-
Multi-metal oxide/TiO 2 anode and Pt/Ti cathode were used in batch processes.
-
77.1% COD removal was achieved with electrooxidation—Fe 2+ activated persulfate.
-
Kinetic models with R 2 values of > 0.80 under all applied current values.






Similar content being viewed by others
References
Agustina F, Bagastyo AY, Nurhayati E (2019) Electro-oxidation of landfill leachate using boron-doped diamond: role of current density, pH and ions. Water Sci Technol 79:921–928. https://doi.org/10.2166/wst.2019.040
Akbari S, Ghanbari F, Moradi M (2016) Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: applying low current density for oxidation mechanism. Chem Eng J 294:298–307. https://doi.org/10.1016/j.cej.2016.02.106
Al-Shamsi MA, Thomson NR (2013) Treatment of a trichloroethylene source zone using persulfate activated by an emplaced nano-Pd-Fe0 zone. Water Air Soil Pollut. https://doi.org/10.1007/s11270-013-1780-1
Amaral MCS, Moravia WG, Lange LC et al (2016) Pilot aerobic membrane bioreactor and nanofiltration for municipal landfill leachate treatment. J Environ Sci Heal Part A Toxic/hazardous Subst Environ Eng 51:640–649. https://doi.org/10.1080/10934529.2016.1159874
Anglada A, Ortiz D, Urtiaga AM, Ortiz I (2010) Electrochemical oxidation of landfill leachates at pilot scale: evaluation of energy needs. Water Sci Technol 61:2211–2217
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association (APHA), the American Water Works Association (AWWA), and the Water Environment Federation (WEF), Washington, DC, USA
Aseman-Bashiz E, Sayyaf H (2020) Metformin degradation in aqueous solutions by electro-activation of persulfate and hydrogen peroxide using natural and synthetic ferrous ion sources. J Mol Liq 300:112285
Bagastyo AY, Novitasari D, Nurhayati E, Direstiyani LC (2020) Impact of sulfate ion addition on electrochemical oxidation of anaerobically treated landfill leachate using boron-doped diamond anode. Res Chem Intermed. https://doi.org/10.1007/s11164-020-04243-3
Barzegar G, Jorfi S, Zarezade V et al (2018) 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 201:370–379. https://doi.org/10.1016/j.chemosphere.2018.02.143
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631
Bu L, Shi Z, Zhou S (2016) Modeling of Fe (II)-activated persulfate oxidation using atrazine as a target contaminant. Sep Purif Technol 169:59–65
Campagna M, Çakmakci M, Yaman FB, Özkaya B (2013) Molecular weight distribution of a full-scale landfill leachate treatment by membrane bioreactor and nanofiltration membrane. Waste Manag 33:866–870
Comninellis C, Kapalka A, Malato S et al (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 83:769–776
Cui Y-H, Xue W-J, Yang S-Q et al (2018) Electrochemical/peroxydisulfate/Fe3+ treatment of landfill leachate nanofiltration concentrate after ultrafiltration. Chem Eng J 353:208–217
Del Moro G, Prieto-Rodriguez L, De Sanctis M et al (2016) Landfill leachate treatment: comparison of standalone electrochemical degradation and combined with a novel biofilter. Chem Eng J 288:87–98
Deng Y, Zhu X, Chen N, Feng C, Wang H, Kuang P, Hu W (2020) Review on electrochemical system for landfill leachate treatment: Performance, mechanism, application, shortcoming, and improvement scheme. Sci Total Environ 745:140768
Devi P, Das U, Dalai AK (2016) In-situ chemical oxidation: principle and applications of peroxide and persulfate treatments in wastewater systems. Sci Total Environ 571:643–657
Ding J, Wang K, Wang S et al (2018a) Electrochemical treatment of bio-treated landfill leachate: Influence of electrode arrangement, potential, and characteristics. Chem Eng J 344:34–41. https://doi.org/10.1016/j.cej.2018.03.043
Ding J, Wei L, Huang H et al (2018b) Tertiary treatment of landfill leachate by an integrated electro-oxidation/electro-coagulation/electro-reduction process: performance and mechanism. J Hazard Mater 351:90–97. https://doi.org/10.1016/j.jhazmat.2018.02.038
El Kateb M, Trellu C, Darwich A et al (2019) Electrochemical advanced oxidation processes using novel electrode materials for mineralization and biodegradability enhancement of nanofiltration concentrate of landfill leachates. Water Res 162:446–455
Fang GD, Dionysiou DD, Wang Y et al (2012) Sulfate radical-based degradation of polychlorinated biphenyls: effects of chloride ion and reaction kinetics. J Hazard Mater 227–228:394–401. https://doi.org/10.1016/j.jhazmat.2012.05.074
Fernandes A, Pacheco MJ, Ciríaco L, Lopes A (2015) Review on the electrochemical processes for the treatment of sanitary landfill leachates: present and future. Appl Catal B Environ 176–177:183–200
Fernandes A, Santos D, Pacheco MJ et al (2016) Electrochemical oxidation of humic acid and sanitary landfill leachate: Influence of anode material, chloride concentration and current density. Sci Total Environ 541:282–291. https://doi.org/10.1016/j.scitotenv.2015.09.052
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62. https://doi.org/10.1016/j.cej.2016.10.064
Ghanbari F, Moradi M, Manshouri M (2014) Textile wastewater decolorization by zero valent iron activated peroxymonosulfate: compared with zero valent copper. J Environ Chem Eng 2:1846–1851
Ghanbari F, Wu J, Khatebasreh M et al (2020) Efficient treatment for landfill leachate through sequential electrocoagulation, electrooxidation and PMS/UV/CuFe2O4 process. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2020.116828
Ghauch A, Tuqan AM, Kibbi N (2015) Naproxen abatement by thermally activated persulfate in aqueous systems. Chem Eng J. https://doi.org/10.1016/j.cej.2015.05.067
He R, Tian BH, Zhang QQ, Zhang HT (2015) Effect of Fenton oxidation on biodegradability, biotoxicity and dissolved organic matter distribution of concentrated landfill leachate derived from a membrane process. Waste Manag 38:232–239. https://doi.org/10.1016/j.wasman.2015.01.006
Hou L, Zhang H, Xue X (2012) Ultrasound enhanced heterogeneous activation of peroxydisulfate by magnetite catalyst for the degradation of tetracycline in water. Sep Purif Technol 84:147–152
Hussain I, Zhang Y, Huang S (2014) Degradation of aniline with zero-valent iron as an activator of persulfate in aqueous solution. Rsc Adv 4:3502–3511
Ilhan F, Kurt U, Apaydin O, Gonullu MT (2008) Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J Hazard Mater 154:381–389. https://doi.org/10.1016/j.jhazmat.2007.10.035
Jaafarzadeh N, Omidinasab M, Ghanbari F (2016) Combined electrocoagulation and UV-based sulfate radical oxidation processes for treatment of pulp and paper wastewater. Process Saf Environ Prot 102:462–472. https://doi.org/10.1016/j.psep.2016.04.019
Jiang M, Ye K, Deng J et al (2018a) Conventional ultrafiltration as effective strategy for dye/salt fractionation in textile wastewater treatment. Environ Sci Technol 52:10698–10708
Jiang M, Ye K, Lin J et al (2018b) Effective dye purification using tight ceramic ultrafiltration membrane. J Memb Sci 566:151–160
Kabuk HA, Ilhan F, Avsar Y et al (2014) Investigation of leachate treatment with electrocoagulation and optimization by response surface methodology. Clean: Soil, Air, Water 42:571–577. https://doi.org/10.1002/clen.201300086
Kjeldsen P, Barlaz MA, Rooker AP et al (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336
Le LuuT (2020) Post treatment of ICEAS-biologically landfill leachate using electrochemical oxidation with Ti/BDD and Ti/RuO2 anodes. Environ Technol Innov. https://doi.org/10.1016/j.eti.2020.101099
Lei Y, Shen Z, Huang R, Wang W (2007) Treatment of landfill leachate by combined aged-refuse bioreactor and electro-oxidation. Water Res 41:2417–2426
Liang C, Bruell CJ, Marley MC, Sperry KL (2004) Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate–thiosulfate redox couple. Chemosphere 55:1213–1223
Lin J, Ye W, Baltaru M-C et al (2016) Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment. J Memb Sci 514:217–228
Liu Z, Guo Y, Shang R et al (2016) A triple system of Fe(III)/sulfite/persulfate: decolorization and mineralization of reactive Brilliant Red X-3B in aqueous solution at near-neutral pH values. J Taiwan Inst Chem Eng 68:162–168. https://doi.org/10.1016/j.jtice.2016.08.027
Liu J, Zhong S, Song Y et al (2018) Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation. J Electroanal Chem 809:74–79
Liu X, Novak JT, He Z (2019) Removal of landfill leachate ultraviolet quenching substances by electricity induced humic acid precipitation and electrooxidation in a membrane electrochemical reactor. Sci Total Environ 689:571–579. https://doi.org/10.1016/j.scitotenv.2019.06.329
Lv X-D, Yang S-Q, Xue W-J et al (2019) Performance of Cu-cathode/Fe3+/peroxymonosulfate process on iohexol degradation. J Hazard Mater 366:250–258
Mandal P, Gupta AK, Dubey BK (2020a) Synthesis of graphite/PbO2 anode: electrodeposition process modeling for improved landfill leachate treatment using RSM and ANN approach. Int J Environ Sci Technol 17:1947–1962. https://doi.org/10.1007/s13762-019-02460-x
Mandal P, Gupta AK, Dubey BK (2020b) Synthesis of graphite/PbO2 anode: electrodeposition process modeling for improved landfill leachate treatment using RSM and ANN approach. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02460-x
Martinez-Huitle CA, Rodrigo MA, Sires I, Scialdone O (2015) Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review. Chem Rev 115:13362–13407
Moon BH, Park YB, Park KH (2011) Fenton oxidation of Orange II by pre-reduction using nanoscale zero-valent iron. Desalination 268:249–252. https://doi.org/10.1016/j.desal.2010.10.036
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ 202:217–261
Norzaee S, Taghavi M, Djahed B, Mostafapour FK (2018) Degradation of Penicillin G by heat activated persulfate in aqueous solution. J Environ Manage 215:316–323
Oh SY, Kim HW, Park JM et al (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168:346–351. https://doi.org/10.1016/j.jhazmat.2009.02.065
Oturan N, Van Hullebusch ED, Zhang H et al (2015) Occurrence and removal of organic micropollutants in landfill leachates treated by electrochemical advanced oxidation processes. Environ Sci Technol 49:12187–12196
Outsiou A, Frontistis Z, Ribeiro RS et al (2017) Activation of sodium persulfate by magnetic carbon xerogels (CX/CoFe) for the oxidation of bisphenol A: process variables effects, matrix effects and reaction pathways. Water Res 124:97–107. https://doi.org/10.1016/j.watres.2017.07.046
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Radjenovic J, Sedlak DL (2015) Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ Sci Technol 49:11292–11302
Rahmani AR, Rezaeivahidian H, Almasi M et al (2016) A comparative study on the removal of phenol from aqueous solutions by electro-Fenton and electro-persulfate processes using iron electrodes. Res Chem Intermed 42:1441–1450. https://doi.org/10.1007/s11164-015-2095-1
Rao YF, Qu L, Yang H, Chu W (2014) Degradation of carbamazepine by Fe(II)-activated persulfate process. J Hazard Mater 268:23–32. https://doi.org/10.1016/j.jhazmat.2014.01.010
Rastogi A, Al-Abed SR, Dionysiou DD (2009) Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl Catal B Environ 85:171–179
Rodriguez S, Vasquez L, Costa D et al (2014) Oxidation of orange G by persulfate activated by Fe (II), Fe (III) and zero valent iron (ZVI). Chemosphere 101:86–92
Segura Y, Martínez F, Melero JA, Fierro JLG (2015) Zero valent iron (ZVI) mediated Fenton degradation of industrial wastewater: treatment performance and characterization of final composites. Chem Eng J 269:298–305. https://doi.org/10.1016/j.cej.2015.01.102
Shao H, Zhao X, Wang Y et al (2017) Synergetic activation of peroxymonosulfate by Co3O4 modified g-C3N4 for enhanced degradation of diclofenac sodium under visible light irradiation. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2017.07.016
Silveira JE, Zazo JA, Casas JA (2019) Coupled heat-activated persulfate-Electrolysis for the abatement of organic matter and total nitrogen from landfill leachate. Waste Manag 97:47–51
Sirés I, Brillas E, Oturan MA et al (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res 21:8336–8367
Soomro GS, Qu C, Ren N et al (2020) Efficient removal of refractory organics in landfill leachate concentrates by electrocoagulation in tandem with simultaneous electro-oxidation and in-situ peroxone. Environ Res 183:109249
Sruthi T, Gandhimathi R, Ramesh ST, Nidheesh PV (2018) Stabilized landfill leachate treatment using heterogeneous Fenton and electro-Fenton processes. Chemosphere 210:38–43. https://doi.org/10.1016/j.chemosphere.2018.06.172
Ukundimana Z, Omwene PI, Gengec E et al (2018) Electrooxidation as post treatment of ultrafiltration effluent in a landfill leachate MBR treatment plant: effects of BDD, Pt and DSA anode types. Electrochim Acta 286:252–263. https://doi.org/10.1016/j.electacta.2018.08.019
Varank G, Guvenc SY, Demir A et al (2020a) Modeling and optimizing electro-persulfate processes using Fe and Al electrodes for paper industry wastewater treatment. Water Sci Technol 81:345–357. https://doi.org/10.2166/wst.2020.115
Varank G, Guvenc SY, Dincer K, Demir A (2020b) Concentrated leachate treatment by electro-fenton and electro-persulfate processes using central composite design. Int J Environ Res 14: 439–461
Varank G, Yazici Guvenc S, Demir A (2020c) Electro-activated peroxymonosulfate and peroxydisulfate oxidation of leachate nanofiltration concentrate: multiple-response optimization. Int J Environ Sci Technol 17:2707–2720. https://doi.org/10.1007/s13762-020-02651-x
Vicente F, Santos A, Romero A, Rodriguez S (2011) Kinetic study of diuron oxidation and mineralization by persulphate: effects of temperature, oxidant concentration and iron dosage method. Chem Eng J. https://doi.org/10.1016/j.cej.2011.03.042
Wang H, Wang Y, Li X et al (2016) Removal of humic substances from reverse osmosis (RO) and nanofiltration (NF) concentrated leachate using continuously ozone generation-reaction treatment equipment. Waste Manag 56:271–279. https://doi.org/10.1016/j.wasman.2016.07.040
Wang H, Li X, Hao Z et al (2017) Transformation of dissolved organic matter in concentrated leachate from nano fi ltration during ozone-based oxidation processes (O 3, O 3 / H 2 O 2 and O 3 / UV ). J Environ Manage 191:244–251. https://doi.org/10.1016/j.jenvman.2017.01.021
Wang Q, Wang B, Ma Y, Xing S (2018) Enhanced superoxide radical production for ofloxacin removal via persulfate activation with Cu-Fe oxide. Chem Eng J 354:473–480. https://doi.org/10.1016/j.cej.2018.08.055
Wang Z, Li J, Tan W et al (2019) Removal of COD from landfill leachate by advanced Fenton process combined with electrolysis. Sep Purif Technol 208:3–11. https://doi.org/10.1016/j.seppur.2018.06.048
Wei X, Gao N, Li C et al (2016) Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem Eng J 285:660–670. https://doi.org/10.1016/j.cej.2015.08.120
Wei Z, Villamena FA, Weavers LK (2017) Kinetics and mechanism of ultrasonic activation of persulfate: an in situ EPR spin trapping study. Environ Sci Technol 51:3410–3417. https://doi.org/10.1021/acs.est.6b05392
Xiong C, Li G, Zhang Z et al (2014) Technique for advanced electrochemical oxidation treatment of nanofiltration concentrate of landfill leachate. Wuhan Univ J Nat Sci 19:355–360. https://doi.org/10.1007/s11859-014-1025-1
Xu Z, Shan C, Xie B et al (2017) Decomplexation of Cu(II)-EDTA by UV/persulfate and UV/H2O2: efficiency and mechanism. Appl Catal B Environ 200:439–447. https://doi.org/10.1016/j.apcatb.2016.07.023
Xue W-J, Cui Y-H, Liu Z-Q et al (2020) Treatment of landfill leachate nanofiltration concentrate after ultrafiltration by electrochemically assisted heat activation of peroxydisulfate. Sep Purif Technol 231:115928
Yang Y, Guo H, Zhang Y, Deng Q (2017) Analysis on the removal of ammonia nitrogen using peroxymonosulfate activated by nanoparticulate zero-valent iron. Chem Pap 71:1497–1505. https://doi.org/10.1007/s11696-017-0144-5
Yang S-Q, Cui Y-H, Liu Y-Y et al (2018) Electrochemical generation of persulfate and its performance on 4-bromophenol treatment. Sep Purif Technol 207:461–469
Yazici Guvenc S, Dincer K, Varank G (2019) Performance of electrocoagulation and electro-Fenton processes for treatment of nanofiltration concentrate of biologically stabilized landfill leachate. J Water Process Eng 31:100863. https://doi.org/10.1016/j.jwpe.2019.100863
Ye W, Liu H, Jiang M et al (2019) Sustainable management of landfill leachate concentrate through recovering humic substance as liquid fertilizer by loose nanofiltration. Water Res 157:555–563. https://doi.org/10.1016/j.watres.2019.02.060
Ye W, Liu R, Chen X et al (2020a) Loose nanofiltration-based electrodialysis for highly efficient textile wastewater treatment. J Memb Sci 608:118182
Ye W, Ye K, Lin F et al (2020b) Enhanced fractionation of dye/salt mixtures by tight ultrafiltration membranes via fast bio-inspired co-deposition for sustainable textile wastewater management. Chem Eng J 379:122321
Yılmaz AE, Dede Sağsöz Y, Sakarya M, Cengiz İ (2020) The investigation of flow rate effect on leachate treatment by electrooxidation process. Sigma J Eng Nat Sci 11:73–82
Zhang H, Wang Z, Liu C et al (2014) Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process. Chem Eng J 250:76–82
Zhang Z, Teng C, Zhou K et al (2020) Degradation characteristics of dissolved organic matter in nanofiltration concentrated landfill leachate during electrocatalytic oxidation. Chemosphere 255:127055
Zhen G, Lu X, Zhao Y et al (2012) Enhanced dewaterability of sewage sludge in the presence of Fe(II)-activated persulfate oxidation. Bioresour Technol 116:259–265. https://doi.org/10.1016/j.biortech.2012.01.170
Zhou B, Yu Z, Wei Q et al (2016) Electrochemical oxidation of biological pretreated and membrane separated landfill leachate concentrates on boron doped diamond anode. Appl Surf Sci 377:406–415. https://doi.org/10.1016/j.apsusc.2016.03.045
Zhou X, Jin W, Chen H et al (2017) Enhancing dewaterability of waste activated sludge by combined oxidative conditioning process with zero-valent iron and peroxymonosulfate. Water Sci Technol 76:2427–2433
Zhou P, Zhang J, Zhang Y et al (2018) Degradation of 2, 4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper. J Hazard Mater 344:1209–1219
Zolfaghari M, Jardak K, Drogui P et al (2016) Landfill leachate treatment by sequential membrane bioreactor and electro-oxidation processes. J Environ Manage 184:318–326. https://doi.org/10.1016/j.jenvman.2016.10.010
Zou X, Zhou T, Mao J, Wu X (2014) Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/persulfate Fenton-like system. Chem Eng J 257:36–44. https://doi.org/10.1016/j.cej.2014.07.048
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision were performed by GV. Investigation and data collection were performed by SYG and EC-G. Analysis and visualization were performed by AC. Software, formal analysis, validation, and methodology were performed by SYG. The first draft of the manuscript was written by GV and EC-G. The manuscript was reviewed and edited by SYG and GV. Electrodes were supplied by BO. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yazici Guvenc, S., Can-Güven, E., Cebi, A. et al. Electro/Fe2+/Persulfate Oxidation of Landfill Leachate Nanofiltration Concentrate Using MMO/TiO2-Ti Anode: A Kinetic Study. Int J Environ Res 15, 959–969 (2021). https://doi.org/10.1007/s41742-021-00365-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-021-00365-7