Abstract
Introduction
A large number of epidemiological studies have revealed that women with endometriosis (EMS) have a higher risk of developing endometriosis-associated ovarian cancer (EAOC). At present, there are few studies on predicting the malignant transformation of ovarian endometriosis (OE). The purpose of this study is to identify and verify the molecules that may be able to predict the malignant transformation of OE.
Methods
The gene expression profiles of ovarian cancer and OE were downloaded from Gene Expression Omnibus (GEO), and a common hub gene ribonucleotide reductase M2 (RRM2) was identified. A total of 44 patients with EAOC and 44 with OE were enrolled in this study. Immunohistochemistry (IHC) and quantitative reverse transcription polymerase chain reaction (RT-qPCR) were used to detect the expression of RRM2, while the relationship between RRM2 and Ki-67 was analyzed by IHC co-localization.
Results
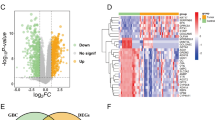
Bioinformatics analysis showed that the expression of RRM2 was low in EMS and high in ovarian cancer. RRM2 was obviously positively expressed in eutopic endometrium (EU), ectopic endometrium (EC), and cancer tissues of EAOC patients. The IHC signal and mRNA levels of RRM2 were higher in the EC of EAOC patients compared with OE patients (P < 0.01). In addition, there was a correlation between the expression of RRM2 and Ki-67 in EC of EAOC patients (P < 0.01).
Conclusion
The upregulated expression of RRM2 in the EC of OE patients may indicate malignant transformation. High expression of RRM2 promotes abnormal proliferation of histiocytes. RRM2 can be used as a potential marker of malignant transformation of OE.





Similar content being viewed by others
References
Schmidt CL. Endometriosis: a reappraisal of pathogenesis and treatment. Fertil Steril. 1985;44(2):157.
Sun Y, Che X, Zhu L, Zhao M, Fu G, Huang X, Xu H, Hu F, Zhang X. Pigment epithelium derived factor inhibits the growth of human endometrial implants in nude mice and of ovarian endometriotic stromal cells in vitro. PLoS ONE. 2012;7(9): e45223.
Kobayashi H, Higashiura Y, Shigetomi H, Kajihara H. Pathogenesis of endometriosis: the role of initial infection and subsequent sterile inflammation (review). Mol Med Rep. 2014;9(1):9–15.
Giudice LC. Endometriosis. N Engl J Med. 2010;362(25):2389–98.
Nishida M, Watanabe K, Sato N, Ichikawa Y. Malignant transformation of ovarian endometriosis. Gynecol Obstet Invest. 2000;50(Suppl 1):18–25.
Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15(1):1–9.
Kobayashi H, Sumimoto K, Kitanaka T, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, Kanayama S, Shigetomi H, Haruta S, Tsuji Y, Ueda S, Terao T. Ovarian endometrioma—risks factors of ovarian cancer development. Eur J Obstet Gynecol Reprod Biol. 2008;138(2):187–93.
Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I. Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int J Clin Oncol. 2009;14(5):383–91.
Guidozzi F. Endometriosis-associated cancer. Climacteric. 2021;1–6.
Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. IARC; 2014.
Kurman RJ, Shih I. The dualistic model of ovarian carcinogenesis. Am J Pathol. 2016;186(4):733–47.
Poole EM, Lin WT, Kvaskoff M, De Vivo I, Terry KL, Missmer SA. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control. 2017;28(5):437–45.
Ness RB. Endometriosis and ovarian cancer: Thoughts on shared pathophysiology. Am J Obstet Gynecol. 2003;189(1):280–94.
Modesitt SC, Tortolero-Luna G, Robinson JB, Gershenson DM, Wolf JK. Ovarian and extraovarian endometriosis-associated cancer. Obstet Gynecol. 2002;100(4):788–95.
Taniguchi F. New knowledge and insights about the malignant transformation of endometriosis. J Obstet Gynaecol Res. 2017;43(7):1093–100.
Jiang L, Zhang M, Wang S, Han Y, Fang X. Common and specific gene signatures among three different endometriosis subtypes. PeerJ. 2020;8: e8730.
Shen J, Yu S, Sun X, Yin M, Fei J, Zhou J. Identification of key biomarkers associated with development and prognosis in patients with ovarian carcinoma: evidence from bioinformatic analysis. J Ovarian Res. 2019. https://doi.org/10.1186/s13048-019-0578-1.
Yang D, He Y, Wu B, Deng Y, Wang N, Li M, Liu Y. Integrated bioinformatics analysis for the screening of hub genes and therapeutic drugs in ovarian cancer. J Ovarian Res. 2020. https://doi.org/10.1186/s13048-020-0613-2.
Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706.
Engstrom Y, Eriksson S, Jildevik I, Skog S, Thelander L, Tribukait B. Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J Biol Chem. 1985;260(16):9114–6.
Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: Their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805(2):141–52.
Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets. 2006;6(5):409.
Aird KM, Zhang G, Li H, Tu Z, Bitler BG, Garipov A, Wu H, Wei Z, Wagner SN, Herlyn M, Zhang R. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013;3(4):1252–65.
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23(8):1539–48.
Itoi T, Sofuni A, Fukushima N, Itokawa F, Tsuchiya T, Kurihara T, Moriyasu F, Tsuchida A, Kasuya K. Ribonucleotide reductase subunit M2 mRNA expression in pretreatment biopsies obtained from unresectable pancreatic carcinomas. J Gastroenterol. 2007;42(5):389–94.
Duxbury MS, Whang EE. RRM2 induces NF-κB-dependent MMP-9 activation and enhances cellular invasiveness. Biochem Biophys Res Commun. 2007;354(1):190–6.
Liu X, Zhou B, Xue L, Yen F, Chu P, Un F, Yen Y. Ribonucleotide reductase subunits M2 and p53R2 are potential biomarkers for metastasis of colon cancer. Clin Colorectal Cancer. 2007;6(5):374–81.
Zhang K, Hu S, Wu J, Chen L, Lu J, Wang X, Liu X, Zhou B, Yen Y. Overexpression of RRM2 decreases thrombspondin-1 and increases VEGF production in human cancer cells in vitro and in vivo: implication of RRM2 in angiogenesis. Mol Cancer. 2009;8(1):11.
Souglakos J, Boukovinas I, Taron M, Mendez P, Mavroudis D, Tripaki M, Hatzidaki D, Koutsopoulos A, Stathopoulos E, Georgoulias V, Rosell R. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer. 2008;98(10):1710–5.
Mazzu YZ, Armenia J, Chakraborty G, Yoshikawa Y, Coggins SA, Nandakumar S, Gerke TA, Pomerantz MM, Qiu X, Zhao H, Atiq M, Khan N, Komura K, Lee GM, Fine SW, Bell C, O’Connor E, Long HW, Freedman ML, Kim B, Kantoff PW. A novel mechanism driving poor-prognosis prostate cancer: Overexpression of the DNA repair gene, ribonucleotide reductase small subunit M2 (RRM2). Clin Cancer Res. 2019;25(14):4480–92.
Aird KM, Li H, Xin F, Konstantinopoulos PA, Zhang R. Identification of ribonucleotide reductase M2 as a potential target for pro-senescence therapy in epithelial ovarian cancer. Cell Cycle. 2013;13(2):199–207.
Acknowledgements
Funding
This work was supported by the Major Science and Technology Projects in Tianjin, grant number 17ZXMFSY00160 and National Natural Science Foundation of China, grant number 81802605. The Rapid Service Fees was funded by the Tianjin Central Hospital of Gynecology Obsterics.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Yuanjing Hu contributed to the study conception and design. Material preparation, data collection and analysis were performed by Binkai Yang. TianWang, NaLi, and Wenwen Zhang contributed to revisions. All authors read and approved the final manuscript.
Disclosures
Yuanjing Hu, Tian Wang, Na Li, Wenwen Zhang and Binkai have nothing to disclosures.
Compliance with Ethics Guidelines
All procedures performed in those studies involving human participants were in accordance with the ethical standards of the local Institutional Review Boards for each site and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, B., Wang, T., Li, N. et al. The High Expression of RRM2 Can Predict the Malignant Transformation of Endometriosis. Adv Ther 38, 5178–5190 (2021). https://doi.org/10.1007/s12325-021-01888-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01888-3