Abstract
Tauopathies are classified according to whether tau deposits predominantly contain tau isoforms with three or four repeats of the microtubule-binding domain. Those in which four-repeat (4R) tau predominates are known as 4R-tauopathies, and include progressive supranuclear palsy, corticobasal degeneration, argyrophilic grain disease, globular glial tauopathies and conditions associated with specific MAPT mutations. In these diseases, 4R-tau deposits are found in various cell types and anatomical regions of the brain and the conditions share pathological, pathophysiological and clinical characteristics. Despite being considered ‘prototype’ tauopathies and, therefore, ideal for studying neuroprotective agents, 4R-tauopathies are still severe and untreatable diseases for which no validated biomarkers exist. However, advances in research have addressed the issues of phenotypic overlap, early clinical diagnosis, pathophysiology and identification of biomarkers, setting a road map towards development of treatments. New clinical criteria have been developed and large cohorts with early disease are being followed up in prospective studies. New clinical trial readouts are emerging and biomarker research is focused on molecular pathways that have been identified. Lessons learned from failed trials of neuroprotective drugs are being used to design new trials. In this Review, we present an overview of the latest research in 4R-tauopathies, with a focus on progressive supranuclear palsy, and discuss how current evidence dictates ongoing and future research goals.
Key points
-
Several lines of evidence substantiate the concept that 4-repeat tauopathies form an aetiologically coherent disease continuum, including progressive supranuclear palsy, corticobasal degeneration, globular glial tauopathy and argyrophilic grain disease.
-
Neurobiological, neuroimaging and neuropathological data suggest that the spread of 4-repeat tau isoforms can induce neurodegeneration and propagation of tau pathology in a unifying disease mechanism.
-
4-repeat tauopathies are increasingly recognized in clinical settings by their characteristic clinical manifestations, spanning from predominantly movement disorders and overlap syndromes to predominantly cognitive syndromes.
-
Biomarkers, such as tau PET ligands, are being developed to substantiate the diagnosis of 4-repeat tauopathies in living patients.
-
Currently, symptomatic therapeutic options for 4-repeat tauopathies are limited, but the available options must not be overlooked.
-
A broad spectrum of 4-repeat tau treatments are currently being developed, making the field of 4-repeat tauopathies an important testbed for precision medicine in neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kovacs, G. G. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol. Appl. Neurobiol. 41, 3–23 (2015).
Spillantini, M. G., Bird, T. D. & Ghetti, B. Frontotemporal dementia and Parkinsonism linked to chromosome 17: a new group of tauopathies. Brain Pathol. 8, 387–402 (1998).
Rosler, T. W. et al. Four-repeat tauopathies. Prog. Neurobiol. 180, 101644 (2019).
Kovacs, G. G. et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 131, 87–102 (2016).
Tacik, P., Sanchez-Contreras, M., Rademakers, R., Dickson, D. W. & Wszolek, Z. K. Genetic disorders with tau pathology: a review of the literature and report of two patients with tauopathy and positive family histories. Neurodegener. Dis. 16, 12–21 (2016).
Stamelou, M., Giagkou, N. & Hoglinger, G. U. One decade ago, one decade ahead in progressive supranuclear palsy. Mov. Disord. 34, 1284–1293 (2019).
Rojas, J. C. & Boxer, A. L. Neurodegenerative disease in 2015: targeting tauopathies for therapeutic translation. Nat. Rev. Neurol. 12, 74–76 (2016).
Weingarten, M. D., Lockwood, A. H., Hwo, S. Y. & Kirschner, M. W. A protein factor essential for microtubule assembly. Proc. Natl Acad. Sci. USA 72, 1858–1862 (1975).
Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–526 (1989).
Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016). A comprehensive overview of tau in health and disease.
Sergeant, N., Wattez, A. & Delacourte, A. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: Tau pathologies with exclusively ‘Exon 10’ isoforms. J. Neurochem. 72, 1243–1249 (1999).
Delacourte, A., Sergeant, N., Wattez, A., Gauvreau, D. & Robitaille, Y. Vulnerable neuronal subsets in Alzheimer’s and Pick’s disease are distinguished by their τ isoform distribution and phosphorylation. Ann. Neurol. 43, 193–204 (1998).
Garcia, M. L. & Cleveland, D. W. Going new places using an old MAP: Tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 13, 41–48 (2001).
Kanaan, N. M. et al. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J. Neurosci. 31, 9858–9868 (2011).
Guo, T., Noble, W. & Hanger, D. P. Roles of tau protein in health and disease. Acta Neuropathol. 133, 665–704 (2017).
Hanger, D. P., Anderton, B. H. & Noble, W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119 (2009).
Smet-Nocca, C. et al. Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol. Biosyst. 7, 1420–1429 (2011).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G. W. & Gong, C. X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 101, 10804–10809 (2004).
Cook, C. et al. Acetylation of the KXGS motifs in tau is a critical determinant in modulation of tau aggregation and clearance. Hum. Mol. Genet. 23, 104–116 (2014).
Cohen, T. J. et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2, 252 (2011).
Alquezar, C., Arya, S. & Kao, A. W. Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front. Neurol. 11, 595532 (2020).
Ghetti, B. et al. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 41, 24–46 (2015).
Chen, Z. et al. Genome-wide survey of copy number variants finds MAPT duplications in progressive supranuclear palsy. Mov. Disord. 34, 1049–1059 (2019).
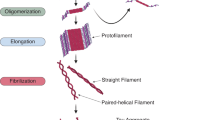
Zhong, Q., Congdon, E. E., Nagaraja, H. N. & Kuret, J. Tau isoform composition influences rate and extent of filament formation. J. Biol. Chem. 287, 20711–20719 (2012).
Adams, S. J., DeTure, M. A., McBride, M., Dickson, D. W. & Petrucelli, L. Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS ONE 5, e10810 (2010).
Schoch, K. M. et al. Increased 4R-tau induces pathological changes in a human-tau mouse model. Neuron 90, 941–947 (2016).
Bunker, J. M., Wilson, L., Jordan, M. A. & Feinstein, S. C. Modulation of microtubule dynamics by tau in living cells: implications for development and neurodegeneration. Mol Biol Cell. 15, 2720–2728 (2004).
Chen, D. et al. Tau local structure shields an amyloid-forming motif and controls aggregation propensity. Nat. Commun. 10, 2493 (2019).
Litvan, I. et al. Environmental and occupational risk factors for progressive supranuclear palsy: case-control study. Mov. Disord. 31, 644–652 (2016).
Caparros-Lefebvre, D. Doparesistant parkinsonism in Guadeloupe and possible relation with the toxicity of isoquinolone derivates. Mov. Disord. 16, 394–395 (2001).
Caparros-Lefebvre, D. & Elbaz, A. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case-control study. Caribbean Parkinsonism Study Group. Lancet 354, 281–286 (1999).
Escobar-Khondiker, M. et al. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J. Neurosci. 27, 7827–7837 (2007).
Hoglinger, G. U. et al. The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. J. Neurochem. 95, 930–939 (2005).
Champy, P. et al. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in guadeloupe. Mov. Disord. 20, 1629–1633 (2005).
Hoglinger, G. U. et al. Experimental evidence for a toxic etiology of tropical parkinsonism. Mov. Disord. 20, 118–119 (2005).
Champy, P. et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 88, 63–69 (2004).
Lannuzel, A. et al. The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience 121, 287–296 (2003).
Hoglinger, G. U. et al. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J. Neurochem. 84, 491–502 (2003).
Alquezar, C. et al. Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci. Rep. 10, 569 (2020).
Caparros-Lefebvre, D. et al. A geographical cluster of progressive supranuclear palsy in northern France. Neurology 85, 1293–1300 (2015).
Yokoyama, J. S. et al. Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol. 133, 825–837 (2017).
Houlden, H. et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56, 1702–1706 (2001).
Caffrey, T. M., Joachim, C., Paracchini, S., Esiri, M. M. & Wade-Martins, R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum. Mol. Genet. 15, 3529–3537 (2006).
Trabzuni, D. et al. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum. Mol. Genet. 21, 4094–4103 (2012).
Li, Y. et al. An epigenetic signature in peripheral blood associated with the haplotype on 17q21.31, a risk factor for neurodegenerative tauopathy. PLoS Genet. 10, e1004211 (2014).
Myers, A. J. et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 25, 561–570 (2007).
Heckman, M. G. et al. Association of MAPT subhaplotypes with risk of progressive supranuclear palsy and severity of tau pathology. JAMA Neurol. 76, 710–717 (2019).
Zhao, N. et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat. Commun. 9, 4388 (2018).
Ghebremedhin, E. et al. Argyrophilic grain disease is associated with apolipoprotein E ε2 allele. Acta Neuropathol. 96, 222–224 (1998).
Tolnay, M., Probst, A., Monsch, A. U., Staehelin, H. B. & Egensperger, R. Apolipoprotein E allele frequencies in argyrophilic grain disease. Acta Neuropathol. 96, 225–227 (1998).
Tolnay, M. & Clavaguera, F. Argyrophilic grain disease: a late-onset dementia with distinctive features among tauopathies. Neuropathology 24, 269–283 (2004).
Togo, T., Cookson, N. & Dickson, D. W. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 12, 45–52 (2002).
Rauch, J. N. et al. LRP1 is a master regulator of tau uptake and spread. Nature 580, 381–385 (2020). An important paper in which LRP1 was identified as the regulator of tau uptake and spread, which may have important therapeutic implications.
Melquist, S. et al. Identification of a novel risk locus for progressive supranuclear palsy by a pooled genomewide scan of 500,288 single-nucleotide polymorphisms. Am. J. Hum. Genet. 80, 769–778 (2007).
Höglinger, G. U. et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705 (2011).
Chen, J. A. et al. Joint genome-wide association study of progressive supranuclear palsy identifies novel susceptibility loci and genetic correlation to neurodegenerative diseases. Mol. Neurodegener. 13, 41 (2018).
Sanchez-Contreras, M. Y. et al. Replication of progressive supranuclear palsy genome-wide association study identifies SLCO1A2 and DUSP10 as new susceptibility loci. Mol. Neurodegener. 13, 37 (2018).
Kouri, N. et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat. Commun. 6, 7247 (2015). GWAS in corticobasal degeneration that links CBD and PSP on the basis of common risk variants.
Bonham, L. W. et al. CXCR4 involvement in neurodegenerative diseases. Transl. Psychiatry 8, 73 (2018).
Jabbari, E. et al. Variation at the TRIM11 locus modifies progressive supranuclear palsy phenotype. Ann. Neurol. 84, 485–496 (2018).
Jabbari, E. et al. Genetic determinants of survival in progressive supranuclear palsy: a genome-wide association study. Lancet Neurol. 20, 107–116 (2021).
Zhao, Y. et al. Appoptosin-mediated caspase cleavage of tau contributes to progressive supranuclear palsy pathogenesis. Neuron 87, 963–975 (2015).
Yuan, S. H. et al. Tauopathy-associated PERK alleles are functional hypomorphs that increase neuronal vulnerability to ER stress. Hum. Mol. Genet. 27, 3951–3963 (2018).
Ferrari, R. et al. Assessment of common variability and expression quantitative trait loci for genome-wide associations for progressive supranuclear palsy. Neurobiol. Aging 35, 1514.e1–1514.e12 (2014).
Allen, M. et al. Gene expression, methylation and neuropathology correlations at progressive supranuclear palsy risk loci. Acta Neuropathol. 132, 197–211 (2016).
Huin, V. et al. The MAPT gene is differentially methylated in the progressive supranuclear palsy brain. Mov. Disord. 31, 1883–1890 (2016).
Smith, P. Y. et al. MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum. Mol. Genet. 20, 4016–4024 (2011).
Tatura, R. et al. microRNA profiling: increased expression of miR-147a and miR-518e in progressive supranuclear palsy (PSP). Neurogenetics 17, 165–171 (2016).
Reyes, J. F., Geula, C., Vana, L. & Binder, L. I. Selective tau tyrosine nitration in non-AD tauopathies. Acta Neuropathol. 123, 119–132 (2012).
Sato, C. et al. Tau kinetics in neurons and the human central nervous system. Neuron 97, 1284–1298.e7 (2018).
Takeda, S. et al. Seed-competent high-molecular-weight tau species accumulates in the cerebrospinal fluid of Alzheimer’s disease mouse model and human patients. Ann. Neurol. 80, 355–367 (2016).
Katsinelos, T. et al. Unconventional secretion mediates the trans-cellular spreading of tau. Cell Rep. 23, 2039–2055 (2018).
Wagshal, D. et al. Divergent CSF τ alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 86, 244–250 (2015).
Barthélemy, N. R. et al. Differential mass spectrometry profiles of tau protein in the cerebrospinal fluid of patients with Alzheimer’s disease, progressive supranuclear palsy, and dementia with lewy bodies. J. Alzheimers Dis. 51, 1033–1043 (2016).
Dujardin, S. et al. Ectosomes: a new mechanism for non-exosomal secretion of Tau protein. PLoS ONE 9, e100760 (2014).
Gerson, J. E. et al. Characterization of tau oligomeric seeds in progressive supranuclear palsy. Acta Neuropathol. Commun. 2, 73 (2014).
Clavaguera, F. et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009). An important study that supports the hypothesis that tau spreading is the mechanism underlying progression, in mice.
Clavaguera, F. et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl Acad. Sci. USA 110, 9535–9540 (2013). An important study that supports the hypothesis that tau spreading is the mechanism underlying progression, using human brain homogenates.
Clavaguera, F., Grueninger, F. & Tolnay, M. Intercellular transfer of tau aggregates and spreading of tau pathology: implications for therapeutic strategies. Neuropharmacology 76, 9–15 (2014).
Goedert, M., Masuda-Suzukake, M. & Falcon, B. Like prions: the propagation of aggregated tau and α-synuclein in neurodegeneration. Brain 140, 266–278 (2017).
Clavaguera, F., Hench, J., Goedert, M. & Tolnay, M. Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol. Appl. Neurobiol. 41, 47–58 (2015).
Hauw, J. J. et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44, 2015–2019 (1994).
Kovacs, G. G. et al. Tauopathies: from molecule to therapy [German]. Nervenarzt 89, 1083–1094 (2018).
Yokota, O. et al. Neuropathological comorbidity associated with argyrophilic grain disease. Neuropathology 38, 82–97 (2018).
Gil, M. J. et al. Argyrophilic grain pathology in frontotemporal lobar degeneration: demographic, clinical, neuropathological, and genetic features. J. Alzheimers Dis. 63, 1109–1117 (2018).
Tatsumi, S. et al. Argyrophilic grains are reliable disease-specific features of corticobasal degeneration. J. Neuropathol. Exp. Neurol. 73, 30–38 (2014).
Ahmed, Z. et al. Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol. 126, 537–544 (2013).
Kovacs, G. G. et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J. Neuropathol. Exp. Neurol. 67, 963–975 (2008).
Rohan, Z., Milenkovic, I., Lutz, M. I., Matej, R. & Kovacs, G. G. Shared and distinct patterns of oligodendroglial response in α-synucleinopathies and tauopathies. J. Neuropathol. Exp. Neurol. 75, 1100–1109 (2016).
Josephs, K. A. et al. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J. Neuropathol. Exp. Neurol. 65, 396–405 (2006).
Tanaka, H. et al. Morphological characterisation of glial and neuronal tau pathology in globular glial tauopathy (types II and III). Neuropathol. Appl. Neurobiol. 46, 344–358 (2020).
Kovacs, G. G. Astroglia and tau: new perspectives. Front. Aging Neurosci. 12, 96 (2020).
Arai, T. et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann. Neurol. 55, 72–79 (2004).
Taniguchi-Watanabe, S. et al. Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol. 131, 267–280 (2016).
Sanders, D. W. et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014).
Narasimhan, S. et al. Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J. Neurosci. 37, 11406–11423 (2017).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Williams, D. R. et al. Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130, 1566–1576 (2007). One of the first studies to show that differences in the underlying distribution and burden of tau pathology explain different PSP phenotypes.
Kovacs, G. G. et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 140, 99–119 (2020).
Kouri, N., Whitwell, J. L., Josephs, K. A., Rademakers, R. & Dickson, D. W. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat. Rev. Neurol. 7, 263–272 (2011).
Ling, H. et al. Fulminant corticobasal degeneration: a distinct variant with predominant neuronal tau aggregates. Acta Neuropathol. 139, 717–734 (2020).
Ling, H. et al. Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 139, 3237–3252 (2016).
Saito, Y. et al. Staging of argyrophilic grains: an age-associated tauopathy. J. Neuropathol. Exp. Neurol. 63, 911–918 (2004).
Robinson, J. L. et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141, 2181–2193 (2018).
Kovacs, G. G. Are comorbidities compatible with a molecular pathological classification of neurodegenerative diseases? Curr. Opin. Neurol. 32, 279–291 (2019).
Koga, S., Lin, W. L., Walton, R. L., Ross, O. A. & Dickson, D. W. TDP-43 pathology in multiple system atrophy: colocalization of TDP-43 and α-synuclein in glial cytoplasmic inclusions. Neuropathol. Appl. Neurobiol. 44, 707–721 (2018).
Jecmenica Lukic, M. et al. Copathology in progressive supranuclear palsy: does it matter? Mov. Disord. 35, 984–993 (2020).
Robinson, J. L. et al. Primary tau pathology, not copathology, correlates with clinical symptoms in PSP and CBD. J. Neuropathol. Exp. Neurol. 79, 296–304 (2020).
Allen, M. et al. Divergent brain gene expression patterns associate with distinct cell-specific tau neuropathology traits in progressive supranuclear palsy. Acta Neuropathol. 136, 709–727 (2018).
Ferrer, I. Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: the forgotten partner. Prog. Neurobiol. 169, 24–54 (2018).
Boluda, S. et al. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 129, 221–237 (2015).
Respondek, G. et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov. Disord. 29, 1758–1766 (2014). One of the largest clinicopathological studies in PSP that identified more phentoypes than previously described.
Armstrong, M. J. et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503 (2013). The first clinical criteria that took into account the phenotypic variability in CBD.
Tsuboi, Y. et al. Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov. Disord. 20, 982–988 (2005).
Ahmed, Z., Josephs, K. A., Gonzalez, J., DelleDonne, A. & Dickson, D. W. Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigro-luysial degeneration and axonal dystrophy. Brain 131, 460–472 (2008).
Ling, H. et al. Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathol. Appl. Neurobiol. 40, 149–163 (2014).
Iankova, V. et al. Video-tutorial for the Movement Disorder Society criteria for progressive supranuclear palsy. Parkinsonism Relat. Disord. 78, 200–203 (2020).
Hoglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The Movement Disorder Society criteria. Mov. Disord. 32, 853–864 (2017). The first MDS–PSP criteria taking into account all described phentoypes in PSP and introducing a ‘suggestive of’ category for early identification of patients.
Respondek, G. et al. Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov. Disord. 32, 995–1005 (2017).
Williams, D. R. et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain 128, 1247–1258 (2005).
Grimm, M. J. et al. How to apply the Movement Disorder Society criteria for diagnosis of progressive supranuclear palsy. Mov. Disord. 34, 1228–1232 (2019).
Kertesz, A. et al. Progressive supranuclear palsy in a family with TDP-43 pathology. Neurocase 21, 178–184 (2015).
de Bruin, V. M., Lees, A. J. & Daniel, S. E. Diffuse Lewy body disease presenting with supranuclear gaze palsy, parkinsonism, and dementia: a case report. Mov. Disord. 7, 355–358 (1992).
Rivaud-Pechoux, S. et al. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology 54, 1029–1032 (2000).
Ling, H. et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 133, 2045–2057 (2010). One of the most comprehensive clinicopathological studies in CBD.
Marsili, L., Dickson, D. W. & Espay, A. J. Globular glial tauopathy may be mistaken for corticobasal syndrome–pointers for the clinician. Mov. Disord. Clin. Pract. 5, 439–441 (2018).
Factor, S. A., Higgins, D. S. & Qian, J. Primary progressive freezing gait: a syndrome with many causes. Neurology 66, 411–414 (2006).
Williams, D. R., Holton, J. L., Strand, K., Revesz, T. & Lees, A. J. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov. Disord. 22, 2235–2241 (2007).
Owens, E. et al. The clinical spectrum and natural history of pure akinesia with gait freezing. J. Neurol. 263, 2419–2423 (2016).
Nagao, S. et al. Progressive supranuclear palsy presenting as primary lateral sclerosis but lacking parkinsonism, gaze palsy, aphasia, or dementia. J. Neurol. Sci. 323, 147–153 (2012).
Liu, A. J. et al. Progressive supranuclear palsy and primary lateral sclerosis secondary to globular glial tauopathy: a case report and a practical theoretical framework for the clinical prediction of this rare pathological entity. Neurocase 26, 91–97 (2020).
Iwasaki, Y. et al. An autopsied case of progressive supranuclear palsy presenting with cerebellar ataxia and severe cerebellar involvement. Neuropathology 33, 561–567 (2013).
Kanazawa, M. et al. Early clinical features of patients with progressive supranuclear palsy with predominant cerebellar ataxia. Parkinsonism Relat. Disord. 19, 1149–1151 (2013).
Ando, S., Kanazawa, M. & Onodera, O. Progressive supranuclear palsy with predominant cerebellar ataxia. J. Mov. Disord. 13, 20–26 (2020).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Josephs, K. A. et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 66, 41–48 (2006).
Josephs, K. A. et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129, 1385–1398 (2006).
Grossman, M. Primary progressive aphasia: clinicopathological correlations. Nat. Rev. Neurol. 6, 88–97 (2010).
Grossman, M. et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. J. Cogn. Neurosci. 8, 135–154 (1996).
Rusina, R. et al. Globular glial tauopathy type I presenting as atypical progressive aphasia, with comorbid limbic-predominant age-related TDP-43 encephalopathy. Front. Aging Neurosci. 11, 336 (2019).
Kim, E. J. et al. Globular glial tauopathy presenting as non-fluent/agrammatic variant primary progressive aphasia with chorea. Parkinsonism Relat. Disord. 44, 159–161 (2017).
Tang-Wai, D. F. et al. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 61, 1134–1135 (2003).
Lee, S. E. et al. Clinicopathological correlations in corticobasal degeneration. Ann. Neurol. 70, 327–340 (2011).
Bak, T. H., Caine, D., Hearn, V. C. & Hodges, J. R. Visuospatial functions in atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 77, 454–456 (2006).
Crutch, S. J. et al. Posterior cortical atrophy. Lancet Neurol. 11, 170–178 (2012).
Grimm, M. J. et al. Clinical conditions “Suggestive of progressive supranuclear palsy”–diagnostic performance. Mov. Disord. 35, 2301–2313 (2020).
Ali, F. et al. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov. Disord. 34, 1144–1153 (2019).
Gazzina, S. et al. Neuropathological validation of the MDS-PSP criteria with PSP and other frontotemporal lobar degeneration. bioRxiv https://doi.org/10.1101/520510 (2019).
Alexander, S. K. et al. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J. Neurol. Neurosurg. Psychiatry 85, 925–929 (2014).
Ouchi, H. et al. Pathology and sensitivity of current clinical criteria in corticobasal syndrome. Mov. Disord. 29, 238–244 (2014).
Respondek, G. et al. Validation of the Movement Disorder Society criteria for the diagnosis of 4-repeat tauopathies. Mov. Disord. 35, 171–176 (2020).
Kato, N., Arai, K. & Hattori, T. Study of the rostral midbrain atrophy in progressive supranuclear palsy. J. Neurol. Sci. 210, 57–60 (2003).
Massey, L. A. et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov. Disord. 27, 1754–1762 (2012). One of very few studies of MRI in definite PSP and MSA.
Paviour, D. C., Price, S. L., Stevens, J. M., Lees, A. J. & Fox, N. C. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 64, 675–679 (2005).
Whitwell, J. L. et al. Disrupted thalamocortical connectivity in PSP: a resting state fMRI, DTI, and VBM study. Parkinsonism Relat. Disord. 17, 599–605 (2011).
Quattrone, A. et al. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 246, 214–221 (2008).
Whitwell, J. L. et al. Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov. Disord. 32, 955–971 (2017). A comprehensive review of imaging in PSP.
Möller, L. et al. Manual MRI morphometry in Parkinsonian syndromes. Mov. Disord. 32, 778–782 (2017).
Surova, Y. et al. Disease-specific structural changes in thalamus and dentatorubrothalamic tract in progressive supranuclear palsy. Neuroradiology 57, 1079–1091 (2015).
Knake, S. et al. In vivo demonstration of microstructural brain pathology in progressive supranuclear palsy: a DTI study using TBSS. Mov. Disord. 25, 1232–1238 (2010).
Agosta, F. et al. Diffusion tensor MRI contributes to differentiate Richardson’s syndrome from PSP-parkinsonism. Neurobiol. Aging 33, 2817–2826 (2012).
Potrusil, T. et al. Diagnostic potential of automated tractography in progressive supranuclear palsy variants. Parkinsonism Relat. Disord. 72, 65–71 (2020).
Seki, M. et al. Diagnostic potential of dentatorubrothalamic tract analysis in progressive supranuclear palsy. Parkinsonism Relat. Disord. 49, 81–87 (2018).
Nigro, S. et al. Track density imaging in progressive supranuclear palsy: a pilot study. Hum. Brain Mapp. 40, 1729–1737 (2019).
Archer, D. B. et al. Development and validation of the Automated Imaging Differentiation in Parkinsonism (AID-P): a multi-site machine learning study. Lancet Digit. Health 1, e222–e231 (2019).
Correia, M. M. et al. Towards accurate and unbiased imaging-based differentiation of Parkinson’s disease, progressive supranuclear palsy and corticobasal syndrome. Brain Commun. 2, fcaa051 (2020).
Morisi, R. et al. Multi-class parkinsonian disorders classification with quantitative MR markers and graph-based features using support vector machines. Parkinsonism Relat. Disord. 47, 64–70 (2018).
Talai, A. S., Sedlacik, J., Boelmans, K. & Forkert, N. D. Widespread diffusion changes differentiate Parkinson’s disease and progressive supranuclear palsy. Neuroimage Clin. 20, 1037–1043 (2018).
Gardner, R. C. et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann. Neurol. 73, 603–616 (2013).
Abos, A. et al. Disrupted structural connectivity of fronto-deep gray matter pathways in progressive supranuclear palsy. Neuroimage Clin. 23, 101899 (2019).
Whitwell, J. L. et al. Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. Neuroimage Clin. 25, 102152 (2020).
Longoni, G. et al. MRI measurements of brainstem structures in patients with Richardson’s syndrome, progressive supranuclear palsy-parkinsonism, and Parkinson’s disease. Mov. Disord. 26, 247–255 (2011).
Agosta, F. et al. The in vivo distribution of brain tissue loss in Richardson’s syndrome and PSP-parkinsonism: a VBM-DARTEL study. Eur. J. Neurosci. 32, 640–647 (2010).
Quattrone, A. et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Parkinsonism Relat. Disord. 54, 3–8 (2018).
Picillo, M. et al. Midbrain MRI assessments in progressive supranuclear palsy subtypes. J. Neurol. Neurosurg. Psychiatry 91, 98–103 (2020).
Caso, F. et al. Progression of white matter damage in progressive supranuclear palsy with predominant parkinsonism. Parkinsonism Relat. Disord. 49, 95–99 (2018).
Nigro, S. et al. Track density imaging: a reliable method to assess white matter changes in progressive supranuclear palsy with predominant parkinsonism. Parkinsonism Relat. Disord. 69, 23–29 (2019).
Hong, J. Y. et al. Comparison of regional brain atrophy and cognitive impairment between pure akinesia with gait freezing and Richardson’s syndrome. Front. Aging Neurosci. 7, 180 (2015).
Nakahara, K. et al. Diagnostic accuracy of MRI parameters in pure akinesia with gait freezing. J. Neurol. 267, 752–759 (2020).
Jabbari, E. et al. Diagnosis across the spectrum of progressive supranuclear palsy and corticobasal syndrome. JAMA Neurol. 77, 377–387 (2020).
Santos-Santos, M. A. et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol. 73, 733–742 (2016).
Whitwell, J. L. et al. An evaluation of the progressive supranuclear palsy speech/language variant. Mov. Disord. Clin. Pract. 6, 452–461 (2019).
Boxer, A. L. et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 63, 81–86 (2006).
Whitwell, J. L. et al. Diffusion tensor imaging comparison of progressive supranuclear palsy and corticobasal syndromes. Parkinsonism Relat. Disord. 20, 493–498 (2014).
Boelmans, K. et al. Diffusion tensor imaging of the corpus callosum differentiates corticobasal syndrome from Parkinson’s disease. Parkinsonism Relat. Disord. 16, 498–502 (2010).
Borroni, B. et al. White matter changes in corticobasal degeneration syndrome and correlation with limb apraxia. Arch. Neurol. 65, 796–801 (2008).
Zhang, Y. et al. Progression of microstructural degeneration in progressive supranuclear palsy and corticobasal syndrome: a longitudinal diffusion tensor imaging study. PLoS ONE 11, e0157218 (2016).
Bharti, K. et al. Abnormal resting-state functional connectivity in progressive supranuclear palsy and corticobasal syndrome. Front. Neurol. 8, 248 (2017).
Ballarini, T. et al. Disentangling brain functional network remodeling in corticobasal syndrome – a multimodal MRI study. Neuroimage Clin. 25, 102112 (2020).
Whitwell, J. L. et al. Imaging correlates of pathology in corticobasal syndrome. Neurology 75, 1879–1887 (2010).
Josephs, K. A. et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer’s disease. Mov. Disord. 25, 1246–1252 (2010).
Josephs, K. A. et al. [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 132, 931–933 (2016).
Akdemir, U. O., Tokcaer, A. B., Karakus, A. & Kapucu, L. O. Brain 18F-FDG PET imaging in the differential diagnosis of parkinsonism. Clin. Nucl. Med. 39, e220–e226 (2014).
Eckert, T. et al. Abnormal metabolic networks in atypical parkinsonism. Mov. Disord. 23, 727–733 (2008).
Garraux, G. et al. Comparison of impaired subcortico-frontal metabolic networks in normal aging, subcortico-frontal dementia, and cortical frontal dementia. Neuroimage 10, 149–162 (1999).
Hosaka, K. et al. Voxel-based comparison of regional cerebral glucose metabolism between PSP and corticobasal degeneration. J. Neurol. Sci. 199, 67–71 (2002).
Juh, R. et al. Cerebral glucose metabolism in corticobasal degeneration comparison with progressive supranuclear palsy using statistical mapping analysis. Neurosci. Lett. 383, 22–27 (2005).
Mishina, M. et al. Midbrain hypometabolism as early diagnostic sign for progressive supranuclear palsy. Acta Neurol. Scand. 110, 128–135 (2004).
Nagahama, Y. et al. Cerebral glucose metabolism in corticobasal degeneration: comparison with progressive supranuclear palsy and normal controls. Mov. Disord. 12, 691–696 (1997).
Salmon, E., Van der Linden, M. V. & Franck, G. Anterior cingulate and motor network metabolic impairment in progressive supranuclear palsy. Neuroimage 5, 173–178 (1997).
Takahashi, R. et al. Brain alterations and Mini-Mental State Examination in patients with progressive supranuclear palsy: voxel-based investigations using 18F-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging. Dement. Geriatr. Cogn. Dis. Extra 1, 381–392 (2011).
Teune, L. K. et al. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov. Disord. 25, 2395–2404 (2010).
Yamauchi, H. et al. Atrophy of the corpus callosum, cognitive impairment, and cortical hypometabolism in progressive supranuclear palsy. Ann. Neurol. 41, 606–614 (1997).
Zwergal, A. et al. Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology 77, 101–109 (2011).
Botha, H. et al. The pimple sign of progressive supranuclear palsy syndrome. Parkinsonism Relat. Disord. 20, 180–185 (2014).
Zalewski, N. et al. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J. Neurol. 261, 710–716 (2014).
Ge, J. et al. Reproducible network and regional topographies of abnormal glucose metabolism associated with progressive supranuclear palsy: multivariate and univariate analyses in American and Chinese patient cohorts. Hum. Brain Mapp. 39, 2842–2858 (2018).
Laureys, S. et al. Fluorodopa uptake and glucose metabolism in early stages of corticobasal degeneration. J. Neurol. 246, 1151–1158 (1999).
Cerami, C. et al. Individual brain metabolic signatures in corticobasal syndrome. J. Alzheimers Dis. 76, 517–528 (2020).
Benvenutto, A. et al. Clinical phenotypes in corticobasal syndrome with or without amyloidosis biomarkers. J. Alzheimers Dis. 74, 331–343 (2020).
Beyer, L. et al. Clinical routine FDG-PET imaging of suspected progressive supranuclear palsy and corticobasal degeneration: a gatekeeper for subsequent Tau-PET imaging? Front. Neurol. 9, 483 (2018).
Tripathi, M. et al. Differential diagnosis of parkinsonian syndromes using F-18 fluorodeoxyglucose positron emission tomography. Neuroradiology 55, 483–492 (2013).
Marti-Andres, G. et al. Multicenter validation of metabolic abnormalities related to PSP according to the MDS-PSP criteria. Mov. Disord. 35, 2009–2018 (2020).
Srulijes, K. et al. Fluorodeoxyglucose positron emission tomography in Richardson’s syndrome and progressive supranuclear palsy-parkinsonism. Mov. Disord. 27, 151–155 (2012).
Park, H. K. et al. Functional brain imaging in pure akinesia with gait freezing: [18F] FDG PET and [18F] FP-CIT PET analyses. Mov. Disord. 24, 237–245 (2009).
Dodich, A. et al. The clinico-metabolic correlates of language impairment in corticobasal syndrome and progressive supranuclear palsy. Neuroimage Clin. 24, 102009 (2019).
Cho, H. et al. Subcortical 18F-AV-1451 binding patterns in progressive supranuclear palsy. Mov. Disord. 32, 134–140 (2017).
Hammes, J. et al. Elevated in vivo [18F]-AV-1451 uptake in a patient with progressive supranuclear palsy. Mov. Disord. 32, 170–171 (2017).
Passamonti, L. et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 140, 781–791 (2017).
Smith, R. et al. Increased basal ganglia binding of 18F-AV-1451 in patients with progressive supranuclear palsy. Mov. Disord. 32, 108–114 (2017).
Whitwell, J. L. et al. [18F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov. Disord. 32, 124–133 (2017).
Whitwell, J. L. et al. Pittsburgh compound B and AV-1451 positron emission tomography assessment of molecular pathologies of Alzheimer’s disease in progressive supranuclear palsy. Parkinsonism Relat. Disord. 48, 3–9 (2018).
Schonhaut, D. R. et al. (18) F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: a multicenter study. Ann. Neurol. 82, 622–634 (2017).
Whitwell, J. L. et al. MRI outperforms [18F]AV-1451 PET as a longitudinal biomarker in progressive supranuclear palsy. Mov. Disord. 34, 105–113 (2019).
Sintini, I. et al. Multimodal neuroimaging relationships in progressive supranuclear palsy. Parkinsonism Relat. Disord. 66, 56–61 (2019).
Cope, T. E. et al. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain 141, 550–567 (2018).
Nicastro, N. et al. (18)F-AV1451 PET imaging and multimodal MRI changes in progressive supranuclear palsy. J. Neurol. 267, 341–349 (2020).
Ghirelli, A. et al. Sensitivity-specificity of tau and amyloid β positron emission tomography in frontotemporal lobar degeneration. Ann. Neurol. 88, 1009–1022 (2020).
McMillan, C. T. et al. Multimodal evaluation demonstrates in vivo 18F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol. 132, 935–937 (2016).
Lowe, V. J. et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol. Commun. 4, 58 (2016).
Marquie, M. et al. Pathologic correlations of [F-18]-AV-1451 imaging in non-Alzheimer tauopathies. Ann. Neurol. 133, 149–151 (2017).
Sander, K. et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement. 12, 1116–1124 (2016).
Brendel, M. et al. 18F-PI-2620 tau-PET in progressive supranuclear palsy – a cross-sectional multi-center study. JAMA Neurol. 77, 1408–1419 (2020).
Kroth, H. et al. Discovery and preclinical characterization of [(18)F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur. J. Nucl. Med. Mol. Imaging 46, 2178–2189 (2019).
Beyer, L. et al. Early-phase [18F]PI-2620 tau-PET imaging as a surrogate marker of neuronal injury. Eur. J. Nucl. Med. Mol. Imaging 47, 2911–2922 (2020).
Endo, H. et al. In vivo binding of a tau imaging probe, [11C]PBB3, in patients with progressive supranuclear palsy. Mov. Disord. 34, 744–754 (2019).
Schroter, N. et al. Tau imaging in the 4-repeat-tauopathies progressive supranuclear palsy and corticobasal syndrome: a 11C-pyridinyl-butadienyl-benzothiazole 3 PET pilot study. Clin. Nucl. Med. 45, 283–287 (2020).
Tagai, K. et al. High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron 109, 42–58.e8 (2021).
Ishiki, A. et al. Tau imaging with [18F]THK-5351 in progressive supranuclear palsy. Eur. J. Neurol. 24, 130–136 (2017).
Brendel, M. et al. [(18)F]-THK5351 PET correlates with topology and symptom severity in progressive supranuclear palsy. Front. Aging Neurosci. 9, 440 (2017).
Schonecker, S. et al. PET imaging of astrogliosis and tau facilitates diagnosis of parkinsonian syndromes. Front. Aging Neurosci. 11, 249 (2019).
Ng, K. P. et al. Monoamine oxidase B inhibitor, selegiline, reduces (18)F-THK5351 uptake in the human brain. Alzheimers Res. Ther. 9, 25 (2017).
Ishiki, A. et al. Neuroimaging-pathological correlations of [(18)F]THK5351 PET in progressive supranuclear palsy. Acta Neuropathol. Commun. 6, 53 (2018).
Cho, H. et al. 18F-AV-1451 binds to motor-related subcortical gray and white matter in corticobasal syndrome. Neurology 89, 1170–1178 (2017).
Kikuchi, A. et al. In vivo visualization of tau deposits in corticobasal syndrome by 18F-THK5351 PET. Neurology 87, 2309–2316 (2016).
Smith, R. et al. In vivo retention of 18F-AV-1451 in corticobasal syndrome. Neurology 89, 845–853 (2017).
Maruyama, M. et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79, 1094–1108 (2013). One of the earliest studies of imaging tau in vivo.
Ezura, M. et al. Longitudinal changes in (18)F-THK5351 positron emission tomography in corticobasal syndrome. Eur. J. Neurol. 26, 1205–1211 (2019).
Ali, F. et al. [(18)F] AV-1451 uptake in corticobasal syndrome: the influence of beta-amyloid and clinical presentation. J. Neurol. 265, 1079–1088 (2018).
Vasilevskaya, A. et al. PET tau imaging and motor impairments differ between corticobasal syndrome and progressive supranuclear palsy with and without Alzheimer’s disease biomarkers. Front. Neurol. 11, 574 (2020).
Hall, S. et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452 (2012).
Saijo, E. et al. 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol. 139, 63–77 (2020).
Abdo, W. F., Bloem, B. R., Van Geel, W. J., Esselink, R. A. & Verbeek, M. M. CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol. Aging 28, 742–747 (2007).
Bridel, C. et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 76, 1035–1048 (2019).
Constantinescu, R., Rosengren, L., Johnels, B., Zetterberg, H. & Holmberg, B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson’s disease and atypical Parkinsonian disorders. Parkinsonism Relat. Disord. 16, 142–145 (2010).
Rojas, J. C. et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann. Clin. Transl. Neurol. 3, 216–225 (2016).
Donker Kaat, L. et al. Serum neurofilament light chain in progressive supranuclear palsy. Parkinsonism Relat. Disord. 56, 98–101 (2018).
Hansson, O. et al. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88, 930–937 (2017).
Magdalinou, N., Lees, A. J. & Zetterberg, H. Cerebrospinal fluid biomarkers in parkinsonian conditions: an update and future directions. J. Neurol. Neurosurg. Psychiatry 85, 1065–1075 (2014).
Magdalinou, N. K. et al. A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 86, 1240–1247 (2015).
Rojas, J. C. et al. CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 90, e273–e281 (2018).
Zetterberg, H. Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron 91, 1–3 (2016).
Boxer, A. L. et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 13, 676–685 (2014). One of the earliest negative neuroprotective trials in PSP, providing important insights for further studies.
Boxer, A. L. et al. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 16, 552–563 (2017).
Lamb, R., Rohrer, J. D., Lees, A. J. & Morris, H. R. Progressive supranuclear palsy and corticobasal degeneration: pathophysiology and treatment options. Curr. Treat. Options Neurol. 18, 42 (2016).
VandeVrede, L., Ljubenkov, P. A., Rojas, J. C., Welch, A. E. & Boxer, A. L. Four-repeat tauopathies: current management and future treatments. Neurotherapeutics 17, 1563–1581 (2020).
Marsili, L., Suppa, A., Berardelli, A. & Colosimo, C. Therapeutic interventions in parkinsonism: corticobasal degeneration. Parkinsonism Relat. Disord. 22, S96–S100 (2016).
Stamelou, M. & Bhatia, K. P. Atypical parkinsonism–new advances. Curr. Opin. Neurol. 29, 480–485 (2016).
Stamelou, M. & Hoglinger, G. A review of treatment options for progressive supranuclear palsy. CNS Drugs 30, 629–636 (2016).
Stamelou, M. & Bhatia, K. P. Atypical parkinsonism: diagnosis and treatment. Neurol. Clin. 33, 39–56 (2015).
Scelzo, E. et al. Peduncolopontine nucleus stimulation in progressive supranuclear palsy: a randomised trial. J. Neurol. Neurosurg. Psychiatry 88, 613–616 (2017).
Moretti, D. V. Available and future treatments for atypical parkinsonism. A systematic review. CNS Neurosci. Ther. 25, 159–174 (2019).
Galazky, I. et al. Deep brain stimulation of the pedunculopontine nucleus for treatment of gait and balance disorder in progressive supranuclear palsy: effects of frequency modulations and clinical outcome. Parkinsonism Relat. Disord. 50, 81–86 (2018).
Dayal, V. et al. Pedunculopontine nucleus deep brain stimulation for parkinsonian disorders: a case series. Stereotact. Funct. Neurosurg. 99, 287–294 (2020).
Kompoliti, K. et al. Pharmacological therapy in progressive supranuclear palsy. Arch. Neurol. 55, 1099–1102 (1998). One of the earliest of few studies of pharmacological treatments in autopsy-confirmed PSP.
Rittman, T., Coyle-Gilchrist, I. T. & Rowe, J. B. Managing cognition in progressive supranuclear palsy. Neurodegener. Dis. Manag. 6, 499–508 (2016).
Litvan, I. et al. Randomized placebo-controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology 57, 467–473 (2001).
Fabbrini, G. et al. Donepezil in the treatment of progressive supranuclear palsy. Acta Neurol. Scand. 103, 123–125 (2001).
Giagkou, N. & Stamelou, M. Emerging drugs for progressive supranuclear palsy. Expert. Opin. Emerg. Drugs 24, 83–92 (2019).
Giagkou, N. & Stamelou, M. Therapeutic management of the overlapping syndromes of atypical parkinsonism. CNS Drugs 32, 827–837 (2018).
Ogawa, T. et al. Prevalence and treatment of LUTS in patients with Parkinson disease or multiple system atrophy. Nat. Rev. Urol. 14, 79–89 (2017).
Batla, A., Tayim, N., Pakzad, M. & Panicker, J. N. Treatment options for urogenital dysfunction in Parkinson’s disease. Curr. Treat. Options Neurol. 18, 45–45 (2016).
Giannantoni, A. et al. Botulinum toxin A for overactive bladder and detrusor muscle overactivity in patients with Parkinson’s disease and multiple system atrophy. J. Urol. 182, 1453–1457 (2009).
Bensimon, G. et al. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 132, 156–171 (2009).
Stamelou, M. et al. In vivo evidence for cerebral depletion in high-energy phosphates in progressive supranuclear palsy. J. Cereb. Blood Flow. Metab. 29, 861–870 (2009).
Stamelou, M. et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov. Disord. 23, 942–949 (2008).
Apetauerova, D. et al. CoQ10 in progressive supranuclear palsy: a randomized, placebo-controlled, double-blind trial. Neurol. Neuroimmunol. Neuroinflamm 3, e266 (2016).
Serenó, L. et al. A novel GSK-3β inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol. Dis. 35, 359–367 (2009).
Tolosa, E. et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov. Disord. 29, 470–478 (2014).
Hoglinger, G. U. et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Mov. Disord. 29, 479–487 (2014).
Leclair-Visonneau, L. et al. Randomized placebo-controlled trial of sodium valproate in progressive supranuclear palsy. Clin. Neurol. Neurosurg. 146, 35–39 (2016).
Min, S. W. et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162 (2015).
VandeVrede, L. et al. Open-label phase 1 futility studies of salsalate and young plasma in progressive supranuclear palsy. Mov. Disord. Clin. Pract. 7, 440–447 (2020).
Tsai, R. M. et al. Reactions to multiple ascending doses of the microtubule stabilizer TPI-287 in patients with Alzheimer disease, progressive supranuclear palsy, and corticobasal syndrome: a randomized clinical trial. JAMA Neurol. 77, 215–224 (2020).
Yanamandra, K. et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann. Clin. Transl. Neurol. 2, 278–288 (2015).
Yanamandra, K. et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80, 402–414 (2013).
Bright, J. et al. Human secreted tau increases amyloid-beta production. Neurobiol. Aging 36, 693–709 (2015).
Boxer, A. L. et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol. 18, 549–558 (2019).
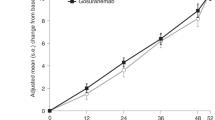
Dam T., et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: a phase 2, randomized, placebo-controlled trial. Nat. Med. https://doi.org/10.1038/s41591-021-01455-x (2020).
West, T. et al. Preclinical and clinical development of ABBV-8E12, a humanized anti-tau antibody, for treatment of Alzheimer’s disease and other tauopathies. J. Prev. Alzheimers Dis. 4, 236–241 (2017).
Höglinger, G. U. et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial. Lancet Neurol. 20, 182–192 (2021).
Courade, J. P. et al. Epitope determines efficacy of therapeutic anti-Tau antibodies in a functional assay with human Alzheimer tau. Acta Neuropathol. 136, 729–745 (2018).
Albert, M. et al. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 142, 1736–1750 (2019).
Novak, P. et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 16, 123–134 (2017).
Zhang, H. et al. Tolfenamic acid inhibits GSK-3β and PP2A mediated tau hyperphosphorylation in Alzheimer’s disease models. J. Physiol. Sci. 70, 29 (2020).
Medina, M. An overview on the clinical development of tau-based therapeutics. Int J Mol. Sci. 19, 1160 (2018).
Sud, R., Geller, E. T. & Schellenberg, G. D. Antisense-mediated exon skipping decreases tau protein expression: a potential therapy for tauopathies. Mol. Ther. Nucleic Acids 3, e180 (2014).
Yuzwa, S. A. et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Chem. Biol. 8, 393–399 (2012).
Ryan, J. M. et al. Phase 1 study in healthy volunteers of the O-GlcNAcase inhibitor ASN120290 as a novel therapy for progressive supranuclear palsy and related tauopathies [abstract O1-12-05]. Alzheimers Dement. 14(7S), 251 (2018).
Piot, I. et al. The progressive supranuclear palsy clinical deficits scale. Mov. Disord. 35, 650–661 (2020).
Brittain, C. et al. Severity dependent distribution of impairments in PSP and CBS: interactive visualizations. Parkinsonism Relat. Disord. 60, 138–145 (2019).
Grötsch, M. T. et al. A modified progressive supranuclear palsy rating scale. Mov. Disord. 36, 1203–1215 (2021).
Lang, A. E. et al. The cortical basal ganglia functional scale (CBFS): development and preliminary validation. Parkinsonism Relat. Disord. 79, 121–126 (2020).
Passamonti, L. et al. [(11)C]PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 90, e1989–e1996 (2018).
Holland, N. et al. Synaptic loss in primary tauopathies revealed by [(11)C]UCB-J positron emission tomography. Mov. Disord. 35, 1834–1842 (2020).
Nieforth, K. & Golbe, L. I. Retrospective study of drug response in 87 patients with progressive supranuclear palsy. Clin. Neuropharmacol. 16, 338–346 (1993).
Muller, J., Wenning, G. K., Wissel, J., Seppi, K. & Poewe, W. Botulinum toxin treatment in atypical parkinsonian disorders associated with disabling focal dystonia. J. Neurol. 249, 300–304 (2002).
Jankovic, J. & Brin, M. F. Therapeutic uses of botulinum toxin. N. Engl. J. Med. 324, 1186–1194 (1991).
Piccione, F., Mancini, E., Tonin, P. & Bizzarini, M. Botulinum toxin treatment of apraxia of eyelid opening in progressive supranuclear palsy: report of two cases. Arch. Phys. Med. Rehabil. 78, 525–529 (1997).
Krack, P. & Marion, M. H. “Apraxia of lid opening,” a focal eyelid dystonia: clinical study of 32 patients. Mov. Disord. 9, 610–615 (1994).
Rohrer, G., Hoglinger, G. U. & Levin, J. Symptomatic therapy of multiple system atrophy. Auton. Neurosci. 211, 26–30 (2018).
Armstrong, M. J. Diagnosis and treatment of corticobasal degeneration topical collection on movement disorders. Curr. Treat. Options. Neurol. 16, 282 (2014).
Bhatia, K. P. & Stamelou, M. Nonmotor features in atypical parkinsonism. Int. Rev. Neurobiol. 134, 1285–1301 (2017).
Gómez-Caravaca, M. T. et al. The use of botulinum toxin in the treatment of sialorrhea in parkinsonian disorders. Neurol. Sci. 36, 275–279 (2015).
Acknowledgements
J.L.W. is supported by the National Institutes of Health (NIH) grants R01-NS89757, R01-DC12519 and R01-DC14942. G.G.K. is supported by the Rossy Foundation, “Rossy PSP Program”, the Edmond J. Safra Foundation and the Bishop Karl Golser Award. G.U.H. was supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy; ID 390857198), DFG (HO2402/18-1), the German Federal Ministry of Education and Research (BMBF, 01KU1403A; 01EK1605A), the EU/EFPIA/Innovative Medicines Initiative [2] Joint Undertaking (IMPRIND grant 116060), the VolkswagenStiftung/Lower Saxony Ministry for Science/Petermax-Müller Foundation (Aetiology and Therapy of Synucleinopathies and Tauopathies).
Author information
Authors and Affiliations
Contributions
M.S. researched data for the article and made substantial contributions to the content. All authors contributed to writing of the manuscript and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks C. Colosimo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Online tool for diagnosis of PSP: https://qxmd.com/calculate/calculator_567/
Supplementary information
Rights and permissions
About this article
Cite this article
Stamelou, M., Respondek, G., Giagkou, N. et al. Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat Rev Neurol 17, 601–620 (2021). https://doi.org/10.1038/s41582-021-00541-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-021-00541-5
This article is cited by
-
Identification of metabolic pathways and key genes associated with atypical parkinsonism using a systems biology approach
Metabolic Brain Disease (2024)
-
Fiber-specific micro- and macroscopic white matter alterations in progressive supranuclear palsy and corticobasal syndrome
npj Parkinson's Disease (2023)
-
Tau PET imaging in progressive supranuclear palsy: a systematic review and meta-analysis
Journal of Neurology (2023)
-
Cell-specific MAPT gene expression is preserved in neuronal and glial tau cytopathologies in progressive supranuclear palsy
Acta Neuropathologica (2023)
-
Dysphagie bei Parkinson-Syndromen
Der Nervenarzt (2023)