Abstract
The thermal behaviour of a natural allanite-(Ce) has been investigated up to 1073 K (at room pressure) by means of in situ synchrotron powder X-ray diffraction and single-crystal neutron diffraction. Allanite preserves its crystallinity up to 1073 K. However, up to 700 K, the thermal behaviour along the three principal crystallographic axes, of the monoclinic β angle and of the unit-cell volume follow monotonically increasing trends, which are almost linear. At T > 700–800 K, a drastic change takes place: an inversion of the trend is observed along the a and b axes (more pronounced along b) and for the monoclinic β angle; in contrast, an anomalous increase of the expansion is observed along the c axis, which controls the positive trend experienced by the unit-cell volume at T > 700–800 K. Data collected back to room T, after the HT experiments, show unit-cell parameters significantly different with respect to those previously measured at 293 K: allanite responds with an ideal elastic behaviour up to 700 K, and at T > 700–800 K its behaviour deviates from the elasticity field. The thermo-elastic behaviour up to 700 K was modelled with a modified Holland–Powell EoS; for the unit-cell volume, we obtained the following parameters: VT0 = 467.33(6) Å3 and αT0(V) = 2.8(3) × 10–5 K−1. The thermal anisotropy, derived on the basis of the axial expansion along the three main crystallographic directions, is the following: αT0(a):αT0(b):αT0(c) = 1.08:1:1.36. The T-induced mechanisms, at the atomic scale, are described on the basis of the neutron structure refinements at different temperatures. Evidence of dehydroxylation effect at T ≥ 848 K are reported. A comparison between the thermal behaviour of allanite, epidote and clinozoisite is carried out.
Similar content being viewed by others
Introduction
Allanite is a sorosilicate, belonging to the epidote group minerals, with general crystal chemical formula A(1)A(2)M(1)M(2)M(3)(SiO4)(Si2O7)O(OH), in which A(1) and A(2) are sites with coordination number CN > 6 and mainly occupied by Ca, and M(1), M(2) and M(3) are octahedral sites (CN = 6), principally occupied by Al and Fe3+ (Dollase 1971; Franz and Liebscher 2004; Armbruster et al. 2006). Epidote group minerals are monoclinic in symmetry, with a structure described in the space group P21/m; however, possible deviations from the expected symmetry were also reported in some epidotes, for example in response to cation ordering (Franz and Liebscher 2004). The main building-block units in the structure of epidotes are: single-silicate tetrahedra (SiO4), double-silicate tetrahedra (Si2O7), and continuous chains of edge-sharing MO6 and MO4(OH)2 octahedra running along [010]. The octahedral chains are bridged by SiO4 and Si2O7 units, as shown in Fig. 1. The crystal chemistry of the epidote group is complex. Clinozoisite (with ideal crystal chemical formula A1,A2Ca2M1,M2,M3Al3(SiO4)(Si2O7)O(OH)) is considered as the reference structure of the group, with three independent octahedral M sites (M1, M2 and M3) fully occupied by Al and the two independent A sites (A1 and A2) occupied by Ca. Following the Commission of the International Mineralogical Association, three different subgroups can be considered (Armbruster et al. 2006). Among those, the allanite subgroup contains rare-earth elements (REE) bearing members, typified by the eponymous mineral “allanite”. It is possible to derive the crystal chemistry of the allanite subgroup minerals from that of clinozoisite, on the basis of coupled homovalent and heterovalent substitutions, as follows:
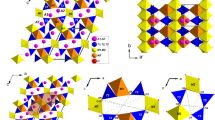
Crystal structure of allanite viewed down [010] and [001], and overlying unit-strain ellipsoid based on Eulerian finite strain calculated between 293 and 673 K [ε2//b, ε1 and ε3 lying in the (010)-plane, \(\varepsilon 1\angle a^\circ = 42.2(5)^\circ\); ε1 = 0.00517(3), ε2 = 0.00324(3), and ε3 = 0.00201(4) K−1, ε1:ε2:ε3 = 2.57:1.61:1]. Tetrahedra (coordinated by Si1, Si2 and Si3 sites) and octahedra (coordinated by the M1, M2 and M3 sites) are shown as closed-faces polyhedra; large spheres represent the A1 and A2 sites. The 8-membered ring of polyhedra (with the diameters O3 ↔ O3, O10 ↔ O6, O6 ↔ O6 and O8 ↔ O8) and the 5-membered ring (with the diameters O3 ↔ O1, O1 ↔ O6 and O9 ↔ O5) are also shown (see text for further details)
with a site population represented by: A1 = M2+, A2 = M3+, M1 = M3+, M2 = M3+, and M3 = M2+. In this light, the general crystal chemical formula of allanite is:
A1(Ca)A2(REE3+)M1,M2(Al)2M3(Fe2+)(SiO4)(Si2O7)O(OH) (Dollase 1971; Bonazzi and Menchetti 1995; Gieré and Sorensen 2004; Armbruster et al. 2006; Bonazzi et al. 2009).
Epidotes occur in diverse geological environments. They mainly occur in low-grade metamorphic rocks (250–400 °C, 0.1–0.2 GPa). However, magmatic epidotes were also reported (Schmidt and Poli 2004). Their stability was observed over a wide range of P–T conditions in geological systems with continental and oceanic crust composition (Poli and Schmidt 1998), influenced even by the Al/Fe3+ ratio, fO2, fluids, etc. (e.g. Holdaway 1972; Liou 1973; Bird and Helgeson 1980; Bird et al. 1988; Klemd 2004). More specifically, allanite occurs as an important accessory mineral in granites and pegmatites (in which it plays an important role on trace element evolution, e.g. Gromet and Silver 1983; Sawka et al. 1984), in high-pressure gneisses (Carswell et al. 2000), in high-pressure garnet-amphibolites (Sorensen 1991), and in eclogites (Tribuzio et al. 1996). On the basis of a multi-methodological study (by experimental petrology, metamorphic petrology and trace element geochemistry), Hermann (2002) described the effect of LREE on the P/T stability field of allanite, along with the experimentally determined element partitioning between allanite and a hydrous granitic melt at 1173 K and 2.0 GPa. Janots et al. (2007) investigated the thermochemistry of a synthetic dissakisite-(La) (i.e. the Mg-counterpart of allanite: CaLaMgAl2(Si2O7)(SiO4)O(OH)), showing how the stability window for dissakisite–clinozoisite lies between 520 and 820 K and 0.1–1.6 GPa, with a significant P/T extension depending on the bulk-rock Ca-content. More recently, Corti et al. (2020) reported how the strain partitioning in host rock can control the LREE release from allanite-(Ce) in subduction zones.
In situ high-pressure (HP) and high-temperature (HT) experiments have mainly been devoted to clinozoisite/zoisite and epidote sensu stricto, to derive thermal and compressional elastic parameters or deformation mechanisms at the atomic scale (e.g. Catti et al. 1988; Holland et al. 1996; Pawley et al. 1996; Comodi and Zanazzi 1997; Franz and Liebscher 2004; Liebscher 2004; Gatta et al. 2010, 2011, 2012; Cámara et al. 2012; Qin et al. 2016; Li et al. 2021). Recently, the compressional behaviour and the main P-mediated deformation mechanisms at the atomic scale of a natural allanite were reported by Gatta et al. (2019), on the basis of an in situ synchrotron single-crystal X-ray diffraction experiment with a diamond anvil cell, providing also a comparative analysis between the P-behaviour of allanite and those of epidote sensu stricto and clinozoisite. This study is the extension of that by Gatta et al. (2019), and we aim at investigate the thermal behaviour and the T-induced structure rearrangement (at the atomic scale) of allanite-(Ce) by in situ synchrotron powder diffraction and single-crystal neutron diffraction. A comparative discussion of the experimental findings on allanite of this study and those previously reported for clinozoisite and epidote sensu stricto will be provided.
Materials and experimental methods
Crystals of allanite from the Alpe Veglia, Divedro valley, Central Alps (Monte Leone nappe), were used in this study. At the Monte Leone nappe, allanite crystals are hosted within pegmatites, involved in the Alpine greenschist metamorphism overprint. The greenschist overprint affected allanite breakdown into monazite and other REE-bearing minerals replacements in retrogressed pegmatites, owing to deformation (up to boudin-like textures) and fluid-assisted reaction. Pegmatite dikes hosting allanite show a strong “NYF” geochemical signature, represented by the occurrence of a unique mineralogy that includes: agardite-(Y), Nb-anatase, cervandonite-(Ce), chernovite-(Y), crichtonite–senaite group minerals, fergusonite-(Y), fluorite, gadolinite-(Y), monazite-(Ce), paraniite-(Y), niobian-rutile, synchysite-(Ce), and xenotime-(Y) (Dal Piaz 1975; Guastoni et al. 2006).
Chemical microanalyses were performed in wavelength-dispersive mode (EPMA-WDS) on a few optically homogeneous millimetric crystals of allanite, using a JEOL JXA-8200 microprobe, at the Earth Sciences Department of the University of Milano (ESD-UniMI). The microprobe was operated as follows: accelerating voltage of 15 kV, beam current of 5 nA, beam diameter of 5 µm, and counting time of 30 s on the peaks and 10 s on the backgrounds. Correction for matrix effects was applied using the PhiRhoZ method, as implemented in the JEOL suite of programs. The following natural and synthetic standards (with spectral lines) were used: grossular (CaKα, SiKα, AlKα), ilmenite (TiKα), fayalite (FeKα), forsterite (MgKα), rodonite (MnKα), metal Co (CoKα), niccolite (AsKα), synthetic YPO4 (YLα, PKα), synthetic ScPO4 (ScLα), synthetic REEPO4 set (LaLα, CeLα, PrLα, NdLα, SmLα, EuLα, GdLα, TbLα, DyLα, HoLα, ErLα, TmLα, YbLα, LuLα), synthetic UO2 (UMβ), synthetic ThO2 (ThMα), and galena (PbMα). The selected crystals of allanite were found to be chemically homogeneous. The average unit-formula, based on (more than) 30 point-analyses and calculated following the protocol recommended by IMA Commission (Armbruster et al. 2006), is (Table 1):
with REE content: (Y0.01La0.16Ce0.28Pr0.03Nd0.15Sm0.03Gd0.02Ho0.01Th0.02)Σ0.72. Allanite from the Alpe Veglia, Monte Leone nappe, is, therefore, rich of La, Ce (dominant) and Nd, among REE.
In situ high-T X-ray diffraction experiments have been performed at the MCX beamline of the ELETTRA synchrotron (Trieste, Italy); the beamline setup description is in Rebuffi et al. (2014). A standard sample of powdered Si (Nist640C) was used for calibration. Crystals of allanite have been previously grinded in an agate mortar and the powders have been loaded in a quartz capillary (300 μm in diameter), which was kept spinning during the data collections. Two ramps have been performed, the first with a monochromatic primary beam 17.504 keV in energy (λ = 0.7083 Å), the second with a primary beam with E = 17.514 keV (λ = 0.7079 Å). The X-ray diffraction patterns have been collected using the high-resolution scintillator detector mounted on the four-circle diffractometer. The same data collection strategy was adopted for both the T-ramps: 2θ range between 3.00° and 17.00°, step-width 0.01°, exposure time 1 s per step. High-temperature conditions were obtained by means of the hot-air gas blower available at the beamline.
The unit-cell parameters of allanite at the different temperatures have been refined adopting a full-profile Rietveld refinement with the structure model of Gatta et al. (2019), using the GSAS-EXPGUI software (Larson and Von Dreele 2004; Toby 2001). During the last cycles, the following parameters have been simultaneously refined: scale factor, background (Chebyshev polynomial, 13 parameters), peak profile (pseudo-Voigt function of Thompson et al. 1988) and unit-cell parameters.
Single-crystal neutron diffraction (SC-ND) data were first collected at room temperature from a crystal of allanite (approx. 4 × 3 × 2 mm3) on the four-circle diffractometer D19 installed at the Institute Laue-Langevin (ILL), Grenoble. The crystal was glued on a vanadium pin with diameter size of 1 mm. Diffraction data were collected with a wavelength of 0.9500 Å, obtained from a 331 Cu monochromator at 2θM = 69.91° take-off angle, and a large two-dimensional area detector was used. The measurement strategy consisted on 5 several ω scans with steps of 0.07° at different χ and φ positions. The Multi-Detector Acquisition Data Software (MAD) from ILL was used for data collection. Unit-cell determination was done using PFIND and DIRAX programs, and processing of the raw data was applied using RETREAT and RAFD19 programs (McIntyre and Stansfield 1988; Wilkinson et al. 1988; Duisenberg 1992). A total number of 9402 reflections were collected (with − 14 ≤ h ≤ + 14, − 10 ≤ k ≤ + 10 and − 18 ≤ l ≤ + 16), out of which 3156 were unique (Rσ = 0.0409, Rint = 0.0705, Laue class 2/m) and 3096 with Io > 2σ (Io), with dmin = 0.54 Å (Table 2). The lattice was found to be metrically monoclinic, and the reflection conditions were found to be consistent with the space group P21/m. Absorption correction was applied, based on composition and shape of the crystal, using the ILL D19ABS program (Matthewman et al. 1982).
Two additional in situ HT SC-ND data collections were performed at 848 and 1023 K, on the four-circle diffractometer D9 at ILL. High temperature was generated using a self-dedicate close shell four-circle vanadium-furnace (uncertainty: ± 1 K). Diffraction data were collected with a wavelength of 0.8360 Å. The measurement strategy consisted of a series of ω-scans or ω-2θ scans for low and high-Q reflections, respectively. The Bragg reflections were collected varying the ω-range as a function of the instrument resolution curve. Intensity integration, background and Lorentz factor correction of the scans were done with the Racer program (written by C. Wilkinson and G. McIntyre, ILL integration program suite, https://forge.ill.fr/projects/sxtalsoft/repository/show/racer). Absorption correction was applied, based on composition and shape of the crystal using the ILL program Datap (the current version of this program is available online on https://forge.ill.fr/). At both the temperatures, the lattice was found to be metrically monoclinic, with reflection conditions consistent with the space group P21/m. The Wilson plot and the statistics of distributions of the normalised structure factors suggest that the structure of allanite is centrosymmetric at 293, 848 and 1023 K. Further details pertaining to the data collected at 848 and 1023 are listed in Table 2.
The anisotropic structure refinements, based on the intensity data collected at room and high temperature, were conducted using the software SHELXL (release 2018–3; Sheldrick 1997, 2008, 2015), starting from the structure model of Gatta et al. (2019), in the space group P21/m. To correct the secondary isotropic extinction effect, the formalism of Larson (1967) was used, as implemented in SHELXL. Neutron scattering lengths of Ca, Mn, Ce, La, Fe, Al, Si, O and H were taken from Sears (1986). Considering the average chemical composition of the allanite of this study, the atomic sites were modelled as follows: the A1 and A2 sites were modelled, respectively, with mixed (Ca + Mn) and (Ca + La) neutron scattering lengths, and the fraction of Ca vs. Mn (A1) and Ca vs. La (A2) were refined; the M1 and M3 octahedral sites as populated by (Fe + Al), and the fraction Fe vs. Al was refined, and the M2 site as populated by Al only; the three independent tetrahedral sites (i.e. Si1, Si2 and Si3) were modelled as fully occupied by Si. Test refinements were conducted using a mixed (Ca + Ce) neutron scattering length to model the A2 site, but the figures of merit were worse than using the (Ca + La) model, likely for the similar scattering length of Ca and Ce (i.e. bCa = 4.70 fm, bCe = 4.84 fm, bLa = 8.24 fm). For all the refinements, convergence was rapidly achieved and, at the end of the last cycles of refinement, no significant correlation was observed in the variance–covariance matrix of the refined parameters. Only for the refinement at 1023 K, to overcome the effects of the high number of reflections with Io < 2σ (Io) on the statistical parameters as calculated in SHELXL, an off-line merging for symmetry with a filter for Io > 2σ (Io) was applied. The principal statistical parameters of the structure refinements are listed in Table 2. Atomic coordinates, site occupancies and displacement parameters are given in Tables 3, 4 and 5. Bond distances and other relevant structural parameters are reported in Table 6.
Results: thermal behaviour of allanite
The evolution of the lattice parameters of allanite with temperature (up to 1073 K) is shown in Fig. 2 and Table 7. Up to 700 K, the thermal behaviour along the three principal crystallographic axes, of the monoclinic β angle and of the unit-cell volume follow monotonically increasing trends, which are almost linear. At T > 700–800 K, a drastic change takes place: an inversion of the trend is observed along the a and b axes (more pronounced along b) and for the monoclinic β angle; in contrast, an anomalous increase of the expansion is observed along the c axis, which somehow governs the positive trend experienced by the unit-cell volume at T > 700–800 K. In addition, data collected back to room T, after the HT experiments up to 1073 K, show unit-cell parameters significantly different with respect to those previously measured at 293 K (Fig. 2; Table 7). We can, therefore, assume that allanite responds with an ideal elastic behaviour up to 700 K, and at T > 700–800 K its behaviour deviates from the elasticity field.
Evolution of the lattice parameters of allanite, normalised to the room T values, with T. Error bars are shorter than the size of the symbols. Dotted lines are guide for the eye. Solid lines represent the modified Holland–Powell Equation of State fit (mHP-EoS) to the experimental data, ranging between the elasticity windows: 293–700 K (zoom diagram, bottom left)
To describe the axial and volume thermal behaviour of allanite within the elasticity domain, a modified Holland and Powell (1998) Equation of State (mHP-EoS) was fitted to the experimental data up to ca. 700 K, using the EoSFit7-GUI program (Angel et al. 2014; Gonzalez-Platas et al. 2016). The mHP-EoS parameters, refined using the data weighted by their uncertainties, are listed in Table 8. For the unit-cell volume, the fit converges to: VT0 = 467.33(6) Å3 and αT0(V) = 2.8(3) × 10–5 K−1 (being α0(V) = 2.7(8) × 10–5 K−1 and α1(V) = − 2.8(9) × 10–4 K−1/2 in mHP-EoS). The thermal anisotropy, derived on the basis of the axial expansion along the three main crystallographic directions, is the following: αT0(a):αT0(b):αT0(c) = 1.08:1:1.36 (Table 8). The monoclinic β-angle value increases linearly with temperature, with: βT/β0 = 0.99991(1) + 3.63(4) × 10−6 T (R2 = 0.9992) (Fig. 2).
Orientation and magnitude of the principal unit-strain components between 293 and 673 K, derived on the basis of the finite Eulerian strain tensor, were calculated using the Win_Strain software (by RJ Angel, www.rossangel.com), with the Cartesian axial system: x//a* and y//b. The strain ellipsoid is oriented with the mid axis (ε2) parallel to the b-axis, and the major (ε1) and minor (ε3) axes dispersed in the (010)-plane: ε1 describes an angle of 42.2 (5)° from a (and consequently 147.2 (5)° from c) (Fig. 1). The elastic anisotropy of allanite, deduced from the unit-strain coefficients between 293 and 673 K, is more pronounced if compared to that obtained considering the principal crystallographic directions, being: ε1 = 0.00517(3) K−1, ε2 = 0.00324(3) K−1, and ε3 = 0.00201(4) K−1, with the resulting anisotropic scheme: ε1:ε2:ε3 = 2.57:1.61:1.
Results: effect of temperature at the atomic scale
The effects of temperature on the crystal structure of allanite-(Ce) are here described starting from the evolution of bond distances and geometry of the coordination polyhedra, based on the single-crystal neutron refinements, as follows:
-
Between 293 and 1023 K, the Si-tetrahedra show only a slight expansion, as deduced from an average increase of bond distances (corrected by “rigid body motion”, Downs et al. 1992; Downs 2000) between 0.01 and 0.02 Å. The polyhedra do not experience any remarkable increase of the distortion. Even the Si1 tetrahedron, which is the most distorted one at room T, shows a difference between the longest and the shortest bond length (Δlmax) that increases only from ~ 0.06 to ~ 0.07 Å.
-
Even the M1, M2 and M3 octahedra show only a modest expansion between 293 and 1023 K, with an increase of bond distances ranging between 0.01 and 0.04 Å. The T-induced distortion is modest: the most distorted octahedron, M3, shows a Δlmax ~ 0.33 Å at 293 K, which increases to ~ 0.37 Å at 1023 K.
-
The bond distances of the A1 and A2 polyhedra increase by 0.01–0.03 Å, between 293 and 1023 K.
The inter-polyhedral changes with T can be described on the basis of the deformation of the 5-membered rings of polyhedra (M2–Si3–M2–Si2–Si1, Fig. 1), confining the cavities in which the A1 site lies, and of the 8-membered rings of polyhedra (M2–Si3–M3–Si2–M2–Si3–M3–Si2), in which the A2 site lies (Fig. 1), acting mainly by polyhedral tilting. The 5-membered ring contains the Si1- and Si2-tetrahedra, bridged by O9: the Si1–O9–Si2 angle increases from 145.56(7)° to 147.5(3)° between 293 and 1023 K. In the same temperature-range, the “diameters” of the ring increase their lengths, though differently: O3 ↔ O1 by ~ 0.035 Å, O6 ↔ O1 by ~ 0.022 Å and O9 ↔ O5 by ~ 0.012 Å (Table 6). Even the diameters of the 8-membered rings of polyhedra change differently in response to the applied temperature: O6 ↔ O10 increases its length by ~ 0.042 Å, O3 ↔ O3 by ~ 0.074 Å, O6 ↔ O6 by ~ 0.030 Å, and O8 ↔ O8 by ~ 0.136 Å (Table 6). The highest directional expansion, observed along the O8 ↔ O8 vector, generates the major effect along [001] and a subordinate effect along [010], and that along O3 ↔ O3 has similar effects on [100] and [001]. However, it is worth to note that some of the aforementioned diameters do not show a monotonic trend with T: the lengths of O6 ↔ O10 (major component along [100], minors along [010] and [001]), O6 ↔ O6 (major component along [100], minors along [010] and [001]) and O9 ↔ O5 (major component along [100], minor along [010]) first increase between 293 and 848 K, and then decrease between 848 and 1023 K; a similar behaviour as that shown along the unit-cell edges a and b with T.
The neutron structure refinement based on the data collected at room T shows a cationic population at the A1, A2, M1, M2 and M3 sites that is compatible, at a first approximation, with the EPMA-WDS data. The refinement converges with: the A1 site populated by ~ 95%Ca + ~ 5%Mn, the A2 site populated by Ca and REE (here modelled with ~ 64%Ca + ~ 36%La), M1 populated by ~ 74%Al + ~ 26%Fe and M3 by ~ 10%Al + ~ 90%Fe, M2 as fully occupied by Al. All the tetrahedral sites are fully occupied by Si. At high temperature, there is no evidence of ordering change at a significant level (≥ 3σ). Only at the M3 site, at 1023 K, the fraction of Fe differs by 7σ from that obtained at the lower temperatures, with a slight decrease of the Fe fraction (i.e. 83% vs. 90%). A potential T-induced Fe migration (and fractionation) to the other octahedral M1 and M2 sites is possible (as observed at high T even by Bonazzi and Menchetti 1994, by ex situ experiments), but the refinement at 1023 K does not reveal this behaviour at a significant level.
One of the additional main effect of temperature on the crystal structure of allanite is represented by the variation of the (anisotropic) displacement parameters magnitude, as shown in Fig. 3 and in Tables 4 and 5, which are expected to be realistic when obtained by neutron diffraction. The calculated root-means-square displacement amplitudes (RMS), expressed in Å in Table 5, show an average increase of ~ 50% between 293 and 848 K, which rises to ~ 100% between 293 and 1023 K. However, the anisotropic scheme of the ellipsoids, expressed by the RMSmax/RMSmin ratio in Table 5, is almost kept with increasing temperature, with a slight decrease of the anisotropy. The most pronounced ellipticity is observed for the O8 and O9 sites (i.e. respectively, with RMSmax/RMSmin of 2.27 and 1.89 at 293 K, and 1.80 and 1.65 at 1023 K). The displacement of O8 reflects its complex bonding configuration: it is the bridging site between Si2 and M3, and is also bonded to two independent A2 sites. The multi-elemental populations at the M3 site (with Al and Fe) and at the A2 site (Ca and REE) is expected to generate a static disorder at the O8 site, with a resulting pronounced displacement parameter. Similar considerations can be extended to the O9 site: it is the bridging oxygen between Si1 and Si2, and is even bonded to two independent A1 sites, having at least a bi-elemental population (Ca and Mn, along with Fe).
H-bonding scheme and evolution of the atomic displacement parameters with T (from left to right: at 293, 848, and 1023 K). Displacement ellipsoids drawn at the 50% probability level. Colour details in Fig. 1
The neutron diffraction data allow us even to describe the effect of temperature on the H-bonding configuration in the structure of allanite. As for epidote sensu stricto, even in allanite there is only one unique H-bond with an energetically favourable configuration, involving O10 as a donor and O4 as an acceptor (i.e. O10–H…O4). According to Kvick et al. (1988) and Gatta et al. (2010), the deviation of the Odonor–H···Oacceptor angle from the linearity (i.e. 180°) is likely ascribable to the electrostatic repulsion generated by trivalent cations at the M3 and A2 sites (at 293 K in this study: M3…H ~ 2.778(2) and A2…H1 ~ 3.042(5) Å). In allanite, the O10–H…O4 angle at 293 K is 169.4(1)°. The almost linear hydrogen bond in zoisite at 15 K (with O10–H…O4 = 176.4(2)°, O10···O4 = 2.742(2) Å and H···O4 = 1.752(2) Å, Smith et al. 1988), corroborates that the presence of Fe3+ at M3 leads to a weakening of the bonds, as reflected by the increase of the Odonor…Oacceptor and H…Oacceptor distances. Beyond the T-induced increase of the displacement ellipsoid magnitude, previously described, the effect of temperature does not change significantly the geometry of the bonding scheme, and no other acceptors are involved in response to the applied T. However, some minor (but significant) changes are observable: between 293 and 1023 K, the H…O4 distance increases (i.e. + 0.111 Å) with a compensational decrease of the O10–H distance (corrected for riding motion effect, i.e. − 0.025 Å); the Odonor…Oacceptor distance (i.e. O10…O4) overall decreases (− 0.075 Å) and the variation of the O10–H…O4 angle is not significant (within the e.s.d.). Test refinements with site occupancy factor (s.o.f.) of the H site free to vary, based on the data at 293, 848 and 1023 K, were performed (Table 3). At 293 K, the s.o.f. is equal to 1.0 within 2σ. At 848 K, the refinement converges either with the s.o.f. fixed to 1.0 or with the refined value of 0.96(1). At 1023 K, the figure of merit is higher with a refined s.o.f. of 0.89(3), showing a significant deviation from the full occupancy. This finding supports a partial dehydroxylation effect, where charge balance is maintained by oxidation of ferrous to ferric iron, as observed in previous ex situ experiments (e.g. Dollase 1973; Bonazzi and Menchetti 1994), according to:
M3Fe2+ + O10OH− → M3Fe3+ + O10O2− + 1/2H2.
Discussion and conclusions
To the best of our knowledge, this is the first study on the thermal expansion of allanite described on the basis of in situ experiments, aimed to provide the thermo-elastic parameters of this mineral and the description of the T-induced structural rearrangement at the atomic scale. The experimental findings of this study confirm that allanite preserves its crystallinity at least up to 1073 K, but behaves elastically likely up to 700 K (Fig. 2, Table 7). Other sorosilicates investigated at high temperature, by in situ X-ray or neutron diffraction experiments, do not show deviation from the elastic behaviour within the T-range investigated (e.g. up to 1173 K for clinozoisite, Pawley et al. 1996; 1023 K for zoisite, Cámara et al. 2012; 1170 K for epidote, Gatta et al. 2011), even up to T-induced breakdown (i.e. 1150–1200 K for strontian piemontite, Catti et al. 1988).
The volume thermal expansion coefficient of allanite (at room conditions), valid within its elasticity window, is αT0(V) = 2.8(3) × 10–5 K−1. Using the same calculation protocol here adopted for allanite (with the mHP-EoS), the coefficients obtained on the basis of the clinozoisite (Pawley et al. 1996) and epidote (Gatta et al. 2011) data are virtually identical: αT0(V) = 2.9(3) × 10–5 K−1 for clinozoisite and αT0(V) = 2.8(2) × 10–5 K−1 for epidote. We cannot exclude that a better precision and accuracy of the data would unveil modest, but significant, differences. Conversely, the (isothermal) bulk compression of these three sorosilicates is significantly different: using a third-order Birch-Murnaghan EoS, the bulk modulus of allanite is KP0,T0 = 131(4) GPa (KP0,T0≡1/βP0,T0) and its P-derivative is K’ = 1.9(8) (Gatta et al. 2019), KP0,T0 = 111(3) GPa and K′ = 7.6(7) for epidote with 0.74 Fe a.p.f.u. (Gatta et al. 2011) and KP0,T0 = 115(2) GPa and K′ = 3.7(2) for epidote with 0.79 Fe a.p.f.u. (Qin et al. 2016), and KP0,T0 = 142(3) GPa and K′ = 5.2(4) for clinozoisite with 0.40 Fe a.p.f.u. (Qin et al. 2016). Epidote is, therefore, the softest member, clinozoisite is the stiffest and allanite lies in between. Comparing the compressional paths of epidotes with different Fe fraction and clinozoisite, Qin et al. (2016) argued how the increasing content of Fe reduces the bulk modulus and increases the first P-derivative, ascribing such a behaviour to the differences in compression in response to the addition of Fe at the M3 site (replacing Al). As discussed by Gatta et al. (2019), the conclusion of Qin et al (2016), confined to the epidote–clinozoisite join, cannot be simplistically extended to allanite, as more variables influence the different behaviour of this mineral: Fe and Al mainly populate at least two octahedral sites (and not only one, as in epidote), and Fe occurs in two different oxidation numbers; Fe, Mn along with a series of other LREE elements (mainly La, Ce, and Nd) replace Ca at the A1 and A2 sites; the A2 site can be partially populated, etc..
The thermo-elastic anisotropy of the allanite here investigated is modest along the three principal crystallographic axes, being αT0(a):αT0(b):αT0(c) = 1.08:1:1.36, but not if we consider magnitude and orientation of the unit-strain ellipsoid, being ε1:ε2:ε3 = 2.57:1.61:1 (with ε2//[010], ε1 and ε3 dispersed in the (010)-plane, ε1∠[100] = 42.2(5)°). At high pressure, the compressional anisotropy of allanite along the three main crystallographic directions is: βP0,T0(a):βP0,T0(b):βP0,T0(c) = 1.23:1:1.52 (recalculated from Gatta et al. 2019). The unit-strain ellipsoid at high pressure is oriented with the mid axis (ε2) parallel to the b-axis, and the major (ε1) and minor (ε3) axes lying in the (010)-plane, with ε1:ε2:ε3 = 2.18:1.47:1 and ε1∠[100] = 136.6(2)° (or 43.4(2)° from a, for an easier comparison with the HT data of this study). These experimental findings show that:
-
Among the three main crystallographic directions, the minimum expansion and compression occurs along [010];
-
However, [010] is not the least expandable or stiffest direction of the structure, as shown by the thermal and compressional unit-strain ellipsoids. The least expandable and stiffest directions, along with the most expandable and softest directions, lie in the (010) plane.
To extend our comparative analysis to epidote (0.74 Fe a.p.f.u., Gatta et al. 2011) and clinozoisite (0.02 Fe a.p.f.u., Holland et al. 1996; Pawley et al. 1996), we have recalculated magnitude and orientation of their unit-strain ellipsoids up to ca. 700 K, adopting the same protocol here applied to allanite. For both epidote and clinozoisite, ε2//[010], ε1 and ε3 are dispersed in the (010) plane, with ε1∠[100] = 57.9(6)° in epidote and ε1∠[100] = 55(3)° in clinozoisite. There is a consistent orientation of the ellipsoids among the three sorosilicates, with a slightly lower ε1∠[100] angle in allanite. This finding suggests that there must be a strong control of the structure topology on the thermal anisotropy, otherwise the three sorosilicates should experience a different anisotropic scheme.
The data on allanite show that the inter- and intra-polyhedral mechanisms, described on the basis of the structure refinements at high temperature, are not sufficient to explain, unambiguously, the magnitude and orientation of the HT unit-strain ellipsoid. It is predictable that the [010] direction cannot be the most expandable one, as the expansion of the structure along this direction is hindered by the presence of the (infinite) edge-sharing M1O6 octahedral chains, running along [010] and acting as “pillars”, so that extension along the chain can only take place through polyhedral expansion (i.e. bond distances extension). On the other hand, it is not trivial to enucleate, on the (010) plane, the reasons for the minor and major expansion directions, which are likely the effect of a complex combination of inter-polyhedral tilting and intra-polyhedral (expansion + distortion), along with cation migration and partial dehydroxylation. Furthermore, it is worth to consider that the HT structure refinements of this study are based on data collected out of the elasticity window (i.e. T > 700 K), whereas the elastic parameters (along with magnitude and orientation of the unit-strain ellipsoid) are valid within the elasticity window (i.e. 293–700 K).
The ex situ high-T experiments on allanite reported by Bonazzi and Menchetti (1994), based on single-crystal X-ray diffraction data, showed how crystals annealed in air for 48 h at several temperatures, ranging between 653 and 1173 K, experienced a significant change of the unit-cell parameters, coupled with relevant structure rearrangements. In particular, in the treated allanite, it was observed an irreversible shortening of the unit-cell edges along a and b, coupled with a lengthening along c and an increase of the monoclinic β angle value. The structure refinements of the treated crystals proved a T-induced oxidation–dehydroxylation/dehydrogenation process, with the mechanism described in the previous section, coupled with a modest migration of octahedral cations (deduced on the basis of the refined electron density at the M sites). Fe-oxidation generates mainly a shortening of the M3–O distances, along with a minor contraction of the M1–O distances, a pronounced lengthening of the Odonor…Oacceptor distance with a compensational shortening of the O10–H distance. The experimental data of the in situ HT experiments of this study cannot be directly compared with the ex situ data of Bonazzi and Menchetti (1994), but the trends observed in both these studies are comparable. Even the in situ experiment of this study shows that, at T higher than the elasticity window (i.e. > 700 K), an inversion of the (ordinary) positive expansion path is observed along the a and b axes (more pronounced along b) and for the monoclinic β angle, whereas an anomalous increase of the expansion is observed along the c axis. Less pronounced are the effects at the atomic scale, likely because the in situ experiment does not reach the same oxidation–dehydroxylation level as that of the ex situ experiments of Bonazzi and Menchetti (1994).
References
Angel RJ, Alvaro M, Gonzalez-Platas J (2014) EosFit7c and a Fortran module (library) for equation of state calculations. Z Kristallogr Cryst Mater 229:405–419
Armbruster T, Bonazzi P, Akasaka M, Bermanec V, Chopin C, Gieré R, Heuss-Assbichler S, Liebscher A, Menchetti S, Pan Y, Pasero M (2006) Recommended nomenclature of epidote-group minerals. Eur J Mineral 18:551–567
Bird DK, Helgeson HC (1980) Chemical interaction of aqueous solutions with epidote-feldspar mineral assemblages in geologic systems, I: thermodynamic analysis of phase relations in the system CaO-FeO-Fe2O3-Al2O3-SiO2-H2O-CO2. Am J Science 280:907–941
Bird DK, Cho M, Janik CJ, Liou JG, Caruso LJ (1988) Compositional, order-disorder, and stable isotopic characteristics of Al-Fe epidote, state 2–14 drill hole, Salton Sea geothermal system. J Geophys Res 93(B11):13135–13144
Bonazzi P, Menchetti S (1994) Structural variations induced by heat treatment in allanite and REE-bearing piemontite. Am Mineral 79:1176–1184
Bonazzi P, Menchetti S (1995) Monoclinic members of the epidote group: effect of the Al↔Fe3+↔Fe2+ substitution and of the entry of REE3+. Mineral Petrol 53:133–153
Bonazzi P, Holtstam D, Bindi L, Nysten P, Capitani GC (2009) Multi-analytical approach to solve the puzzle of an allanite-subgroup mineral from Kesebol, Västra Götaland, Sweden. Am Mineral 94:121–134
Cámara F, Gatta GD, Meven M, Pasqual D (2012) Thermal expansion and high temperature structure evolution of zoisite by single-crystal X-ray and neutron diffraction. Phys Chem Minerals 39:27–45
Carswell DA, Wilson RN, Zhai M (2000) Metamorphic evolution, mineral chemistry and thermobarometry of schists and orthogneisses hosting ultra-high pressure eclogites in the Dabieshan of central China. Lithos 52:121–155
Catti M, Ferraris G, Ivaldi G (1988) Thermal behaviour of the crystal structure of strontian piemontite. Am Mineral 73:1370–1376
Comodi P, Zanazzi PF (1997) The pressure behaviour of clinozoisite and zoisite. An X-ray diffraction study. Am Mineral 82:61–68
Corti L, Zanoni D, Gatta GD, Zucali M (2020) Strain partitioning in host rock controls light rare earth element release from allanite- (Ce) in subduction zones. Mineral Mag 84:93–118
Dal Piaz G (1975) Angelo Bianchi. La val Devero ed i suoi minerali. Memorie Istituto Geologia Università Padova, allegato Volume X, Società Cooperativa, Tipografica, Padova, Italy
Dollase WA (1971) Refinement of the crystal structure of epidote, allanite and hancockite. Am Mineral 56:447–464
Dollase WA (1973) Mössbauer spectra and iron distribution in the epidote-group minerals. Z Kristallogr 138:41–63
Downs RT (2000) Analysis of harmonic displacement factors. In: Hazen RM, Downs RT (eds) High-temperature and high-pressure crystal chemistry. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, vol 41, Washington, pp 61–117
Downs RT, Gibbs GV, Bartelmehs KL, Boisen MB Jr (1992) Variations of bond lengths and volumes of silicate tetrahedra with temperature. Am Mineral 77:751–757
Duisenberg AJM (1992) Indexing in single-crystal diffractometry with an obstinate list of reflections. J Appl Cryst 25:92–96
Franz G, Liebscher A (2004) Physical and chemical properties of epidote minerals—an introduction. In: Franz G, Liebscher A (eds) Epidotes. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, vol 56, Washington, USA, pp 1–81
Gatta GD, Meven M, Bromiley G (2010) Effects of temperature on the crystal structure of epidote: a neutron single-crystal diffraction study at 293 and 1,070 K. Phys Chem Minerals 37:475–485
Gatta GD, Merlini M, Lee Y, Poli S (2011) Behavior of epidote at high pressure and high temperature: a powder diffraction study up to 10 GPa and 1,200 K. Phys Chem Minerals 38:419–428
Gatta GD, Alvaro M, Bromiley G (2012) A low temperature X-ray single-crystal diffraction and polarised infra-red study of epidote. Phys Chem Minerals 39:1–15
Gatta GD, Milani S, Corti L, Comboni D, Lotti P, Merlini M, Liermann H-P (2019) Allanite at high pressure: effect of REE on the elastic behaviour of epidote-group minerals. Phys Chem Minerals 46:783–793
Gieré R, Sorensen SS (2004) Allanite and other REE-rich epidote-group minerals. In: Franz G, Liebscher A (eds) Epidotes. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, vol 56, Washington, USA, pp 431–493
Gonzalez-Platas J, Alvaro M, Nestola F, Angel RJ (2016) EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching. J Appl Cryst 49:1377–1382
Gromet LP, Silver LT (1983) Rare earth element distributions among minerals in a granodiorite and their petrogenetic implications. Geochim Cosmochim Acta 47:925–940
Guastoni A, Pezzotta F, Vignola P (2006) Characterization, paragenesis and genetic inferences of arsenates, sulfates and vanadates of Fe, Cu, Pb, Zn from Mount Cervandone (Western Alps, Italy). Period Mineral 76:141–150
Hermann J (2002) Allanite: thorium and light rare earth element carrier in subducted crust. Chem Geol 192:289–306
Holdaway MJ (1972) Thermal stability of Al-Fe epidotes as a function of fO2 and Fe content. Contrib Min Petrol 37:307–340
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
Holland TJB, Redfern SAT, Pawley AR (1996) Volume behaviour of hydrous minerals at high pressure and temperature: II. Compressibilities of lawsonite, zoisite, clinozoisite, and epidote. Am Mineral 81:341–348
Klemd R (2004) Fluid inclusions in epidote minerals and fluid development in epidote-bearing rocks. In: Franz G, Liebscher A (eds) Epidotes. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, vol 56, Washington, USA, pp 197–234
Kvick Å, Pluth JJ, Richardson JW Jr, Smith JV (1988) The ferric ion distribution and hydrogen bonding in epidote: a neutron diffraction study at 15 K. Acta Cryst B44:351–355
Larson AC (1967) Inclusion of secondary extinction in least-squares calculations. Acta Cryst 23:664–665
Larson AC, Von Dreele RB (2004) General structure analysis system (GSAS), Los Alamos National Laboratory Report LAUR, pp 86–748
Li B, Xu J, Zhang D, Ye Z, Huang S, Fan D, Zhou W, Xie H (2021) Thermoelasticity and stability of natural epidote at high pressure and high temperature: implications for water transport during cold slab subduction. Geosci Front 12:921–928
Liebscher A (2004) Spectroscopy of epidote minerals. In: Franz G, Liebscher A (eds) Epidotes. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, vol 56, Washington, USA, pp 125–170
Liou JG (1973) Synthesis and stability relations of epidote, Ca2Al2FeSi3O12 (OH). J Petrol 14:381–413
Matthewman JC, ThompsonP BPJ (1982) The Cambridge crystallography subroutine library. J Appl Cryst 15:167–173
McIntyre GJ, Stansfield RFD (1988) A general Lorentz correction for single-crystal diffractometers. Acta Cryst A44:257–262
Pawley AR, Redfern SAT, Holland TJB (1996) Volume behaviour of hydrous minerals at high pressure and temperature: 1. Thermal expansion of lawsonite, zoisite, clinozoisite, and diaspore. Am Mineral 81:335–340
Poli S, Schmidt MW (1998) The high-pressure stability of zoisite and phase relationships of zoisite-bearing assemblages. Contrib Mineral Petrol 130:162–175
Qin F, Wu X, Wang Y, Fan D, Qin S, Yang K, Townsend JP, Jacobsen SD (2016) High-pressure behavior of natural single-crystal epidote and clinozoisite up to 40 GPa. Phys Chem Minerals 43:649–659
Rebuffi L, Plaisier JR, Abdellatief M, Lausi A, Scardi P (2014) MCX: a synchrotron radiation beamline for X-ray diffraction line profile analysis. Z Anorg Allg Chem 640:3100–3106
Sawka WN, Chappell BW, Norrish K (1984) Light-rare-earth element zoning in sphene and allanite during granitoid fractionation. Geology 12:131–134
Schmidt MW, Poli S (2004) Magmatic epidotes. In: Franz G, Liebscher A (eds) Epidotes. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America and Geochemical Society, vol 56, Washington, pp 399–430
Sears VF (1986) Neutron scattering lengths and cross-sections. In: Sköld K, Price DL (eds) Neutron scattering, methods of experimental physics, 23A. Academic Press, Cambridge, pp 521–550
Sheldrick GM (1997) SHELX-97. Programs for crystal structure determination and refinement. University of Göttingen, Germany
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8
Smith JV, Pluth JJ, Richardson JW Jr, Kvick Å (1988) Neutron diffraction study of zoisite at 15 K and X-ray study at room temperature. Z Kristallogr 179:305–321
Sorensen SS (1991) Petrogenetic significance of zoned allanite in garnet amphibolites from a paleo-subduction zone: Catalina Schist, southern California. Am Mineral 76:589–601
Thompson P, Cox DE, Hastings JB (1988) Rietveld refinement of Debye-Scherrer synchrotron X-ray data from Al2O3. J Appl Cryst 20:79–83
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Cryst 34:210–213
Tribuzio R, Messiga B, Vannucci R, Bottazzi P (1996) Rare earth element redistribution during high-pressure low-temperature metamorphism in ophiolitic Fe-gabbros (Liguria, northwestern Italy): implications for light REE mobility in subduction zones. Geology 24:711–714
Wilkinson C, Khamis HW, Stansfield RFD, McIntyre GJ (1988) Integration of single-crystal reflections using area multidetectors. J Appl Cryst 21:471–478
Acknowledgements
ELETTRA (Basovizza, Trieste, Italy) and ILL (Grenoble, France, metadata repository, https://doi.org/10.5291/ILL-DATA.EASY-658) are acknowledged for provision of beamtime. G.D.G., F.P. and P.L. acknowledge the support of the Italian Ministry of Education (MIUR) through the project “Dipartimenti di Eccellenza 2018–2022”. G.D.G. and P.L. acknowledge the support of the University of Milano through the project “Piano di Sostegno alla Ricerca 2019”. The authors thank two anonymous reviewers and the editor C. McCammon for the revision of the manuscript. This paper is dedicated to Friedrich A. Seifert (b. 1941), Professor emeritus, BGI—Universität Bayreuth, on the occasion of his 80th birthday.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gatta, G.D., Pagliaro, F., Lotti, P. et al. Allanite at high temperature: effect of REE on the thermal behaviour of epidote-group minerals. Phys Chem Minerals 48, 32 (2021). https://doi.org/10.1007/s00269-021-01154-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-021-01154-6