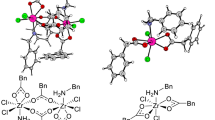
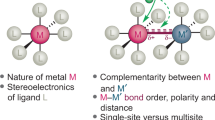
Abstract
Cooperative multimetallic catalysis is a fascinating field of research further expanding the role of homogeneous catalysis. The simultaneous activation of two substrates or functionalities by different catalytic entities and their subsequent coupling are the responsible for the rate acceleration and selectivity exerted by these systems. The nature of the metal moieties is the responsible of the activation step, while selectivity mainly derives from supramolecular interactions between the substrates and the catalysts, particularly during coupling. The control of both activation and coupling opens the way for the design of new highly selective synthetic routes proceeding in mild conditions. Molecular modelling is becoming nowadays an essential tool in rational design of metal-mediated catalysis. Theoretical models provide fundamental insights on the substrate activation by metals as well as on the interactions between the different catalysts. This can allow the deciphering at the atomic level of the preferential pathways leading to different reaction products. In this contribution, we discuss a series of representative examples of multimetallic cooperative processes described by means of molecular modelling.













Similar content being viewed by others
References
Ghosh AC, Duboc C, Gennari M (2021) Synergy between metals for small molecule activation: Enzymes and bio-inspired complexes. Coord Chem Rev 428:213606. https://doi.org/10.1016/j.ccr.2020.213606
Campos J (2020) Bimetallic cooperation across the periodic table. Nat Rev Chem 4:696–702. https://doi.org/10.1038/s41570-020-00226-5
Romiti F, del Pozo J, Paioti PHS, Gonsales SA, Li X, Hartrampf FWW, Hoveyda AH (2019) Different strategies for designing dual-catalytic enantioselective processes: from fully cooperative to non-cooperative systems. J Am Chem Soc 141:17952–17961. https://doi.org/10.1021/jacs.9b05464
Kim UB, Jung DJ, Jeon HJ, Rathwell K, Lee S-G (2020) Synergistic Dual Transition Metal Catalysis. Chem Rev 120:13382–13433. https://doi.org/10.1021/acs.chemrev.0c00245
Sperger T, Sanhueza IA, Kalvet I, Schoenebeck F (2015) Computational studies of synthetically relevant homogeneous organometallic catalysis involving Ni, Pd, Ir, and Rh: an overview of commonly employed dft methods and mechanistic insights. Chem Rev 115:9532–9586. https://doi.org/10.1021/acs.chemrev.5b00163
Sameera WMC, Maseras F (2012) Transition metal catalysis by density functional theory and density functional theory/molecular mechanics. WIREs Comput Mol Sci 2:375–385. https://doi.org/10.1002/wcms.1092
Harvey JN, Himo F, Maseras F, Perrin L (2019) Scope and challenge of computational methods for studying mechanism and reactivity in homogeneous catalysis. ACS Catal 9:6803–6813. https://doi.org/10.1021/acscatal.9b01537
Lledós A (2021) Computational organometallic catalysis: where we are, where we are going. Eur J Inorg Chem 26:2547–2555. https://doi.org/10.1002/ejic.202100330
Koch W, Holthausen MC (2015) A chemist’s guide to density functional theory. John Wiley & Sons, NJ
Hohenberg P, Kohn W (1964) Inhomogeneous Electron Gas. Physical Review 136:B864–B871. https://doi.org/10.1103/PhysRev.136.B864
Grimme S (2012) Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem Eur J 18:9955–9964. https://doi.org/10.1002/chem.201200497
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Phys Chem 132:154104. https://doi.org/10.1063/1.3382344
Besora M, Vidossich P, Lledós A, Ujaque G, Maseras F (2018) calculation of reaction free energies in solution: a comparison of current approaches. J Phys Chem A 122:1392–1399. https://doi.org/10.1021/acs.jpca.7b11580
Luchini G, Alegre-Requena JV, Funes-Ardoiz I, Paton RS (2020) GoodVibes: automated thermochemistry for heterogeneous computational chemistry data. F1000Research 9:291. https://doi.org/10.12688/f1000research.22758.1
Pedregal JRG, Funes-Ardoiz I, Sciortino G, Sánchez-Aparicio JE, Ujaque G, Lledós A, Maréchal JD, Maseras F (2019) GARLEEK: Adding an extra flavor to ONIOM. J Comput Chem 40:381–386. https://doi.org/10.1002/jcc.25612
Chung LW, Sameera WMC, Ramozzi R, Page AJ, Hatanaka M, Petrova GP, Harris TV, Li X, Ke Z, Liu F, Li H-B, Ding L, Morokuma K (2015) The ONIOM method and its applications. Chem Rev 115:5678–5796. https://doi.org/10.1021/cr5004419
Thiel W (2014) Semiempirical quantum–chemical methods. WIREs Comput Mol Sci 4:145–157. https://doi.org/10.1002/wcms.1161
Christensen AS, Kubař T, Cui Q, Elstner M (2016) Semiempirical quantum mechanical methods for noncovalent interactions for chemical and biochemical applications. Chem Rev 116:5301–5337. https://doi.org/10.1021/acs.chemrev.5b00584
Grimme S, Bannwarth C, Shushkov P (2017) A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-Block Elements (Z = 1–86). J Chem Theory Comput 13:1989–2009. https://doi.org/10.1021/acs.jctc.7b00118
Stewart JJP (2013) Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J Mol Model 19:1–32. https://doi.org/10.1007/s00894-012-1667-x
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Klamt A, Schüürmann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc Perkin Trans 2:799–805. https://doi.org/10.1039/P29930000799
Besora M, Braga AAC, Ujaque G, Maseras F, Lledós A (2011) The importance of conformational search: a test case on the catalytic cycle of the Suzuki-Miyaura cross-coupling. Theor Chem Acc 128:639–646. https://doi.org/10.1007/s00214-010-0823-6
Seminario JM (1996) Calculation of intramolecular force fields from second-derivative tensors. Int J Quantum Chem 60:1271–1277
Li P, Merz KM (2016) MCPB.py: A Python Based Metal Center Parameter Builder. J Chem Inf Model 56:599–604. https://doi.org/10.1021/acs.jcim.5b00674
Zheng S, Tang Q, He J, Du S, Xu S, Wang C, Xu Y, Lin F (2016) VFFDT: A New Software for Preparing AMBER force field parameters for metal-containing molecular systems. J Chem Inf Model 56:811–818. https://doi.org/10.1021/acs.jcim.5b00687
Vidossich P, Lledós A, Ujaque G (2016) First-principles molecular dynamics studies of organometallic complexes and homogeneous catalytic processes. Acc Chem Res 49:1271–1278. https://doi.org/10.1021/acs.accounts.6b00054
Marx D, Hutter J (2009) Ab Initio Molecular Dynamics: Basic Theory and Advanced Methods. Cambridge University Press, Cambridge
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density-functional theory. Phys Rev Lett 55:2471–2474. https://doi.org/10.1103/PhysRevLett.55.2471
Vidossich P, Magistrato A (2014) QM/MM molecular dynamics studies of metal binding proteins. Biomolecules 4:616
Besora M, Maseras F (2018) Microkinetic modeling in homogeneous catalysis. WIREs Comput Mol Sci 8:e1372. https://doi.org/10.1002/wcms.1372
Jaraíz, M., (2020) DFT-based microkinetic simulations: a bridge between experiment and theory in synthetic chemistry. New Directions in the Modeling of Organometallic Reactions 81–105.
Weinhold, F., Natural Bond Orbital Methods. In Encyclopedia of Computational Chemistry, 1998.
Bader RF (1985) Atoms in molecules. Acc Chem Res 18:9–15
Fernández I, Bickelhaupt FM (2014) The activation strain model and molecular orbital theory: understanding and designing chemical reactions. Chem Soc Rev 43:4953–4967. https://doi.org/10.1039/C4CS00055B
Daver H, Harvey JN, Rebek J, Himo F (2017) Quantum chemical modeling of cycloaddition reaction in a self-assembled capsule. J Am Chem Soc 139:15494–15503. https://doi.org/10.1021/jacs.7b09102
Sciortino G, Lledós A, Vidossich P (2019) Bonding rearrangements in organometallic reactions: from orbitals to curly arrows. Dalton Trans 48:15740–15752. https://doi.org/10.1039/C9DT03063H
Zhang J-X, Sheong FK, Lin Z (2018) Unravelling Chemical Interactions with Principal Interacting Orbital Analysis. Chem Eur J 24:9639–9650. https://doi.org/10.1002/chem.201801220
Zhang J-X, Sheong FK, Lin Z (2020) Principal interacting orbital: A chemically intuitive method for deciphering bonding interaction. WIREs Comput. Mol. Sci. 10:e1469. https://doi.org/10.1002/wcms.1469
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) NCIPLOT: A program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Funes-Ardoiz I, Maseras F (2018) Oxidative coupling mechanisms: current state of understanding. ACS Catal 8:1161–1172. https://doi.org/10.1021/acscatal.7b02974
Ueura K, Satoh T, Miura M (2007) Rhodium- and iridium-catalyzed oxidative coupling of benzoic acids with alkynes via regioselective C−H Bond Cleavage. J Org Chem 72:5362–5367. https://doi.org/10.1021/jo070735n
Ueura K, Satoh T, Miura M (2007) An efficient waste-free oxidative coupling via regioselective C−H bond cleavage: Rh/Cu-catalyzed reaction of benzoic acids with alkynes and acrylates under Air. Org Lett 9:1407–1409. https://doi.org/10.1021/ol070406h
Funes-Ardoiz I, Maseras F (2016) Cooperative reductive elimination: the missing piece in the oxidative-coupling mechanistic puzzle. Angew Chem Int Ed 55:2764–2767. https://doi.org/10.1002/anie.201510540
Funes-Ardoiz I, Maseras F (2018) Computational characterization of the mechanism for the oxidative coupling of benzoic acid and alkynes by rhodium/copper and rhodium/silver systems. Chem Eur J 24:12383–12388. https://doi.org/10.1002/chem.201800627
Rivada-Wheelaghan O, Comas-Vives A, Fayzullin RR, Lledós A, Khusnutdinova JR (2020) Dynamic PdII/CuI multimetallic assemblies as molecular models to study metal-metal cooperation in sonogashira coupling. Chem Eur J 26:12168–12179. https://doi.org/10.1002/chem.202002013
Martínez de Salinas S, Mudarra ÁL, Benet-Buchholz J, Parella T, Maseras F, Pérez-Temprano MH (2018) New Vistas in Transmetalation with Discrete “AgCF3” Species: Implications in Pd-Mediated Trifluoromethylation Reactions. Chem Eur J 24:11895–11898. https://doi.org/10.1002/chem.201802586
Feng W, Wang T, Liu D, Wang X, Dang Y (2019) Mechanism of the Palladium-Catalyzed C(sp3)–H arylation of aliphatic amines: unraveling the crucial role of Silver(I) Additives. ACS Catal 9:6672–6680. https://doi.org/10.1021/acscatal.9b01412
Wu C, McCollom SP, Zheng Z, Zhang J, Sha S-C, Li M, Walsh PJ, Tomson NC (2020) Aryl fluoride activation through palladium-magnesium bimetallic cooperation: a mechanistic and computational study. ACS Catal 10:7934–7944. https://doi.org/10.1021/acscatal.0c01301
Yoshikai N, Matsuda H, Nakamura E (2009) Hydroxyphosphine ligand for nickel-catalyzed cross-coupling through nickel/magnesium bimetallic cooperation. J Am Chem Soc 131:9590–9599. https://doi.org/10.1021/ja903091g
Yoshikai N, Mashima H, Nakamura E (2005) Nickel-catalyzed cross-coupling reaction of aryl fluorides and chlorides with grignard reagents under nickel/magnesium bimetallic cooperation. J Am Chem Soc 127:17978–17979. https://doi.org/10.1021/ja056327n
Bhaskararao B, Singh S, Anand M, Verma P, Prakash P, C, A., Malakar, S., Schaefer, H. F., Sunoj, R. B., (2020) Is silver a mere terminal oxidant in palladium catalyzed C-H bond activation reactions? Chem Sci 11:208–216. https://doi.org/10.1039/C9SC04540F
Wang J, Zhan L, Wang G, Wei Y, Shi M, Zhang J (2020) Pd-Promoted cross coupling of iodobenzene with vinylgold via an unprecedented phenyl transmetalation from Pd to Au. Chem Commun 56:6213–6216. https://doi.org/10.1039/D0CC02645J
Fromm A, van Wüllen C, Hackenberger D, Gooßen LJ (2014) J Am Chem Soc 136:10007–10023. https://doi.org/10.1021/ja503295x
Deolka S, Rivada-Wheelaghan O, Aristizábal SL, Fayzullin RR, Pal S, Nozaki K, Khaskin E, Khusnutdinova JR (2020) Metal–metal cooperative bond activation by heterobimetallic alkyl, aryl, and acetylide PtII/CuI complexes. Chem Sci 11:5494–5502. https://doi.org/10.1039/D0SC00646G
Zhang H, Hatzis GP, Dickie DA, Moore CE, Thomas CM (2020) Redox chemistry and H-atom abstraction reactivity of a terminal zirconium(iv) oxo compound mediated by an appended cobalt(i) center. Chem Sci 11:10729–10736. https://doi.org/10.1039/D0SC04229C
Ye J, Cammarota RC, Xie J, Vollmer MV, Truhlar DG, Cramer CJ, Lu CC, Gagliardi L (2018) Rationalizing the reactivity of bimetallic molecular catalysts for CO2 Hydrogenation. ACS Catal 8:4955–4968. https://doi.org/10.1021/acscatal.8b00803
Musgrave CB, Zhu W, Coutard N, Ellena JF, Dickie DA, Gunnoe TB, Goddard WA (2021) Mechanistic studies of styrene production from benzene and ethylene using [(η2-C2H4)2Rh(μ-OAc)]2 as catalyst precursor: identification of a Bis-RhI Mono-CuII complex as the catalyst. ACS Catal 11:5688–5702. https://doi.org/10.1021/acscatal.1c01203
Kalek M, Himo F (2017) Mechanism and selectivity of cooperatively catalyzed meyer-schuster rearrangement/tsuji–trost allylic substitution. evaluation of synergistic catalysis by means of combined DFT and kinetics simulations. J Am Chem Soc 139:10250–10266. https://doi.org/10.1021/jacs.7b01931
Zamani F, Babaahmadi R, Yates BF, Gardiner MG, Ariafard A, Pyne SG, Hyland CJT (2019) Dual gold-catalyzed cycloaromatization of unconjugated (E)-Enediynes. Angew Chem Int Ed 58:2114–2119. https://doi.org/10.1002/anie.201810794
Acknowledgements
We thank for financial support the CERCA Programme/Generalitat de Catalunya and MINECO (Grant PID2020-112825RB-I00). G.S. thanks Spanish MINECO Juan de la Cierva program, FJC2019-039135-I. Prof. Agustí Lledós is kindly acknowledged for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sciortino, G., Maseras, F. Computational Study of Homogeneous Multimetallic Cooperative Catalysis. Top Catal 65, 105–117 (2022). https://doi.org/10.1007/s11244-021-01493-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01493-2