Abstract
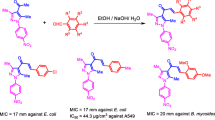
A series of novel hybrids of indole-pyrimidine moieties were synthesized and evaluated for their in vitro anti-cancer, in vitro anti-bacteria and in vitro anti-fungal activities. The results showed that most of these compounds possessed significant cytotoxic potency against four cancer cell lines, HeLa, HEK 293T and MCF-7. The compounds 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine,4-(4-chlorophenyl)-6-(1-methyl-1H-indol-3-yl) pyrimidin-2-amine and4-(1H-indole-3-yl)-6-phenylpyrimidin-2-amine showed good activity against HEK 293T. The compounds 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine and 4-(4-chlorophenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine showed good to moderate activity against MCF-7, whereas compound 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine exhibit moderate activity against HaLa S3 cell line. The newly synthesized derivatives were also screened for their in vitro anti-bacterial activity against Bacillus subtilis, B. megatherium, B. pumilis, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter aerogenes, Streptococcus pyogenes, Staphylococcus aureus, Proteus vulgaris, Escherichia coli, using streptomycin as a standard drug. Among tested compound 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine shows more potent activity compared to standard, where as the remaining analogues exhibited well to moderate activity compared to standard. Anti-fungal screening results suggest that the compound 4-(4-chlorophenyl)-6-(1H-indol-3-yl)pyrimidin-2-amineshowed potent activity against Dreschleria halides. The remaining compounds showed nearest activity against all the tested fungal strains compared to standard drug.
Similar content being viewed by others
REFERENCES
Unger-Saldaña, K., World. J. Clin. Oncol., 2014, vol. 5, pp. 465–477. https://doi.org/10.5306/wjco.v5.i3.465
Mora-Bermúdez, F. and Huttner, W.B., Mol. Biol. Cell., 2015, vol. 26, pp. 4302–4306. https://doi.org/10.1091/mbc.E15-03-0152
Kaur, R., Kaur, G., Gill, R.K., Soni, R., and Bariwal, J., Eur. J. Med. Chem., 2014, vol. 87, pp. 89–124. https://doi.org/10.1016/j.ejmech.2014.09.051
Driowya, M., Leclercq, M.J., Verones, V., Barczyk, A.M., Lecoeur, N., Renault, N., Flouquet, A., Ghinet P., and Berthelot, N., Eur. J. Med. Chem., 2016, vol. 115, pp. 393–405. https://doi.org/10.1016/j.ejmech.2016.03.056
Z. Gan-Or, G.A., Rouleau, E.E., and Benarroch, Neurology, 2016, vol. 87, pp. 2173–2184. https://doi.org/10.1212/wnl.0000000000003444
Kumar, A.S., Reddy, M.A., Jain, N., Kishor, C., Murthy, T.R., Ramesh, B.D., Supriya, B., Addlagatta, A., Kalivendi, S.V., and Sreedhar, B., Eur. J. Med. Chem., 2013, vol. 60, pp. 305–324. https://doi.org/10.1016/j.ejmech.2012.12.008
Strzyz, P., Nat. Rev. Mol. Cell Biol., 2016, vol. 17, pp. 333–341. https://doi.org/10.1038/nrm.2016.63
Dumontet, C. and Jordan, M.A., Nat. Rev. Drug Discov., 2010, vol. 9, pp. 790–803. https://doi.org/10.1038/nrd3253
Shelke, S.H., Mhaske, P.C., Kasam, S.K., and Bobade, V.D., J. Heterocycl. Chem., 2014, vol. 51, pp. 1893–1897. https://doi.org/10.1002/jhet.1910
Singh, P., Eur. J. Med. Chem., 2014, vol. 74, pp. 440–450. https://doi.org/10.1016/j.ejmech.2013.12.047
Sharma, S.K., Kumar, P., Narasimhan, B., Ramasamy, K., Mani, V., Mishra, K.R., Majeed, A., Eur. J. Med. Chem., 2012, vol. 48, pp. 16–25. https://doi.org/10.1016/j.ejmech.2011.11.028
Mehndiratta, S., Hsieh, Y.L., Liu, Y.M., and Wang, A.W., Eur. J. Med. Chem., 2014, vol. 85, pp. 468–479. https://doi.org/10.1016/j.ejmech.2014.08.020
Liew, L.P.P., Fleming, J., Longeon, A., Mouray, E., and Florent, I., Tetrahedron, 2014, vol. 70, pp. 4910–4920. https://doi.org/10.1016/j.tet.2014.05.068
Yamuna, E., Kumar, R.A., and Zeller, M., Eur. J. Med. Chem., 2012, vol. 47, pp. 228–238. https://doi.org/10.1016/j.ejmech.2011.10.046
Comín-Anduix, B., Agell, N., Bachs, O., and Ovadi, J., Mol. Pharmacol., 2001, vol. 60, pp. 1235–1242. https://doi.org/10.1124/mol.60.6.1235
Nassar, E., J. Am. Sci., 2010, vol. 6, pp. 463–471. https://doi.org/10.1134/S0003683810050017
Nagarapu, L., Vanaparthi, S., Bantu, R., and Kumar, C.G., Eur. J. Med. Chem., 2013, vol. 69, pp. 817–822. https://doi.org/10.1016/j.ejmech.2013.08.024
Pingxian Liu, Yang Yang, Yunxiang Tang, Tao Yang, Zitai Sang, and Zhiyong Liu, Eur. J. Med. Chem., 2019, vol. 163, pp. 169–182. https://doi.org/10.1016/j.ejmech.2018.11.054
Ballell, A.F., Robert, G.A.C., and Chung Young, R., Bioorg. Med. Chem. Lett., 2007, vol. 17, pp. 1736–1740. https://doi.org/10.1016/j.bmcl.2006.12.066
Gorlitzer, K., Herbig, S., and Walter, R.D., Org. Chem. Int., 1997, vol. 52, pp. 670–672. https://doi.org/10.1155/2013/582079
Malik, V., Singh, P., and Kumar, S., Tetrahedron, 2006, vol. 62, pp. 5944–5951. https://doi.org/10.1016/j.tet.2006.04.018
Al-Issa, A.S., Saudi Pharm. J., 2013, vol. 21, pp. 305–316. https://doi.org/10.1016/j.jsps.2012.09.002
Wagner, E., Al-Kadasi, K., Zimecki, M., and Sawka-Dobrowolska, W., Eur. J. Med. Chem., 2008, vol. 43, pp. 2498–2504. https://doi.org/10.1016/j.ejmech.2008.01.035
Miyazaki, Y., Matsunaga, S., Tang J., Maeda, Y., Nakano, M., and Philippe, R.J., Bioorg. Med. Chem. Lett., 2005, vol. 15, pp. 2203–2207. https://doi.org/10.1016/j.bmcl.2005.03.034
Christopherson, R.I. and Lyons, S.D., Med. Res. Rev., 1990, vol. 10, pp. 505–548. https://doi.org/10.1002/med.2610100406
Raffa, D., Daidone, G., Maggio, B., and Cascioferro S., II Farmaco Sci., 2004, vol. 59, pp.451–455. https://doi.org/10.1016/j.farmac.2003.10.006
Yang, W., Ruan, Z., Wang, Y., Van-Kirk, K., Ma, Z., and Arey, B.J., J. Med. Chem., 2009, vol. 52, pp. 1204–1208. https://doi.org/10.1021/jm801178c
Hanselmann, R., Job, G.E., Johnson, G., Lou, R.L., and Martynow, J.G., Org. Process Res. Dev., 2010, vol. 14, p. 152. https://doi.org/10.1021/op900252a
Nikhila, G., Dayakumar, D., and Manjunatha Kumsi., J. Saudi Chem. Soc., 2017, vol. 7, pp. 761–775. https://doi.org/10.1016/j.jscs.2015.09.003
Biradar, J.S. and Somappa, S.B., Pharm. Lett., 2012, vol. 4, pp. 344–348. http://scholarsresearchlibrary.com/archive.html
Tanveer Mahamad Alli, S. and Habtamu D., J. Chem., 2020, vol. 2020, pp. 1–9. https://doi.org/10.1155/2020/4358453
Mohamed, M.S., Youns, M.M., and Ahmed, N.M., Med. Chem. Res., 2014, vol. 23, pp. 3374–3388. https://doi.org/10.1007/s00044-014-0916-1
Akue-Gedu, R., Debiton, E., Ferandin, Y., and Meijer, L.M., Bioorg. Med. Chem., 2009, vol. 17, pp. 4420–4424. https://doi.org/10.1016/j.bmc.2009.05.017
Radwan, M.A. and El-Sherbiny, M., Bioorg. Med. Chem., 2007, vol. 15, pp. 1206–1211. https://doi.org/10.1016/j.bmc.2006.11.023
Selvam, T.P., James, C.R., Dniandev, P.V., and Valzita, S.K., Res. Pharm., 2012, vol. 2, pp. 01–09. https://doi.org/10.24959/ophcj.14.804
Mosmann, T., J., Immunol. Methods, 1983, vol. 65, pp. 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
National Committee for Clinical Laboratory, Nat. Commun. Clin. Lab. Stands., 1982, vol. 33, p. 242.
Romagnoli, R., Prencipe, F., Lopez-Cara, L.C., and Oliva, P., J. Enzyme Inhib. Med. Chem., 2018, vol. 33, pp. 727–742. https://doi.org/10.1080/14756366.2018.1450749
ACKNOWLEDGMENTS
The authors are thankful to the director of Indian Institute of Chemical Technology in Hyderabad for providing spectral data, thankful to chairman (Chaitanya Deemed to be University) for providing financial support, Warangal. The authors are thankful to the head, Department of Bio-technology, Kakatiya University Warangal for providing data of biological activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Juluru Bhaskar, Srinivas, B., Gouthami, D. et al. One-Pot Multi-Component Synthesis and Biological Evaluation of Novel Indole-Pyrimidine Derivatives as Potent Anti-Cancer and Anti-Microbial Agents. Russ J Bioorg Chem 47, 954–962 (2021). https://doi.org/10.1134/S106816202104004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202104004X