Abstract
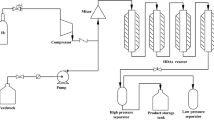
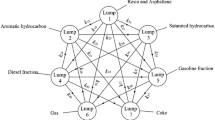
Based on the experimental data of full-range middle-low temperature coal tar (LTCT) hydrodeoxidation (HDO), the phenolic compounds (PC) in feedstock were divided into three lumps, four lumps or five lumps according to different reaction activities. Three corresponding lumping kinetic models of LTCT HDO were established. The Levenberg–Marguardt algorithm was used to calculate the kinetic parameters of each model. The fitting results of the three models are in good agreement with the experimental data, as the average relative error is less than 1.01%. Three types of lumped dynamic models are compared in detail, and the five-lumped model has an average relative error of less than 0.91%. Hence, this model can reliably predict PC removal and showed a good extrapolation performance. The effects of liquid hourly space velocity (LHSV), initial hydrogen pressure (P) and reaction temperature (T) on the HDO process of LTCT were analyzed by five-lumped model calculation, and the reaction law of HDO was obtained. The results show that the order of influence of various factors on the oxygen content of hydrogenated oil is LHSV > P ≥ T. Moreover, the hydrogenation process conditions were optimized by simulation: T = 663 K, P = 14 MPa, LHSV = 0.3 h−1 and V(H2)/V(Oil) of 1100:1, under which the expected oxygen content was 468.28 μg g−1. The results of this study can also provide a scientific understanding of HDO mechanism, greatly reduce the number of LTCT industrial tests, reduce the oil inspection frequency, and help to improve the industrial hydrogenation process.









Similar content being viewed by others
Abbreviations
- LTCT:
-
Middle-low temperature coal tar
- HDO:
-
Hydrodeoxygenation
- PC:
-
Phenolic compounds
- T :
-
Reaction temperature (K)
- P :
-
Initial hydrogen pressure (MPa)
- LHSV :
-
Liquid hourly space velocity (h−1)
- V(H2)/V(Oil):
-
Volume ratio of hydrogen under standard conditions to the volume of feed oil
- t:
-
Reaction time (h)
- xi :
-
The ratio of lump-i oxygen content to total oxygen content in the feedstock (%)
- ki0 :
-
Pre-exponential factor of Arrhenius equation of lump-i (h−1)
- ki :
-
Apparent reaction rate constant of lump-i
- w0 :
-
Oxygen content in feed (μg g−1)
- W:
-
Oxygen content in product (μg g−1)
- wi0 :
-
Oxygen content of lump-i in feed (μg g−1)
- wi :
-
Oxygen content of lump-i in product (μg g−1)
- Ei :
-
Activation energy of lump-i (kJ mol−1)
- ai :
-
Correction factor for reaction pressure of lump-i
- bi :
-
Correction factor for reaction time of lump-i
- di :
-
Oxygen adsorption self-blocking parameter
- ni :
-
Reaction order
- R:
-
Universal gas constant (kJ mol−1 K−1)
- \(\overline{{\mathrm{E}}_{\mathrm{r}}}\) :
-
The average relative error (%)
- SSR:
-
Sum of Squares due to residuals
- \({\mathrm{w}}^{\mathrm{Cal}}\) :
-
Calculated oxygen content of experimental sample i (μg g−1)
- \({\mathrm{w}}^{\mathrm{Exp}}\) :
-
Experimental oxygen content of experimental sample i (μg g−1)
- \({\mathrm{y}}_{\mathrm{i}}^{\mathrm{Cal}}\) :
-
Calculated deoxidation rate of experimental sample i (%)
- \({\mathrm{y}}_{\mathrm{i}}^{\mathrm{Exp}}\) :
-
Experimental deoxidation rate of experimental sample i (%
References
Song QQ, Mu Y, HouWangZheng YCJY (2020) Comparative analysis of the strength of petrochemical industry between China and USA. Chem Ind Eng Prog 39:1607–1619
Qian MG, Miao XX, Xu JL (2007) Green mining of coal resources harmonizing with environment. J China Coal Soc 32:1–7
Brandt AR, Millard-Ball A, Ganser M (2013) Peak oil demand: the role of fuel efficiency and alternative fuels in a global oil production decline. Environ Sci Technol 47:8031–8041
Nolan P, Shipman A, Rui H (2004) Coal liquefaction, Shenhua Group, and China’s energy security. Eur Manag J 22:150–164
Yang Y, Li D, Zhang L (2016) Development, status, and prospects of coal tar hydrogenation technology. Energy Technol-Ger 4:1338–1348
Fan X, Li D, Feng X (2020) Modelling and simulation of industrial trickle bed reactor hydrotreating for whole fraction low-temperature coal tar simultaneous hydrodesulfurisation and hydrodenitrification. Fuel 269:117362
Change Y (2007) Comparison and economic analysis of coal tar hydrogenation technology, Master Dissertation, Northwest University
Li D, Li Z, Li W (2013) Hydrotreating of low temperature coal tar to produce clean liquid fuels. J Anal Appl Pyrol 100:245–252
Liu X, Li D, Feng X (2019) Kinetics study and reactor simulation of full-range low-temperature coal tar during hydrodeoxygenation process. J Anal Appl Pyrol 41:2725–2733
Pan L (2020) Study on hydrodeoxygenation and co-hydrogenation process of coal tar and bio-oil, PhD Dissertation, Northwest University
Zhan F, Lv Z, Wang L (2000) Identification of phenolic compounds in LCO S and their effects on stability of diesels. J Fuel Chem Technol 28:59–62
Hara T, Jones L, Li NC (1981) Ageing of src liquids. Fue 60:1143–1148
Leckel D (2006) Catalytic hydroprocessing of coal-derived gasification residues to fuel blending stocks: effect of reaction variables and catalyst on hydrodeoxygenation (hdo), hydrodenitrogenation (hdn), and hydrodesulfurization (hds). Energy Fuels 20:1761–1766
Laurent E, Delmon B (2002) Influence of oxygen-, nitrogen-, and sulfur-containing compounds on the hydrodeoxygenation of phenols over sulfided cobalt-molybdenum/.gamma.-alumina and nickel-molybdenum/.gamma.-alumina catalysts. Ind Eng Chem Res 32:2516–2524
Odebunmi EO, Ollis DF (1983) Catalytic hydrodeoxygenation. I. Conversions of o-, p-, and m-cresols. Chem Inform 80:56–64
Weigold H (1982) Behaviour of Co-Mo-Al2O3 catalysts in the hydrodeoxygenation of phenols. Fuel 61:1021–1026
Moreau C, Aubert C, Moreau C (1988) Structure-activity relationships in hydroprocessing of aromatic and heteroaromatic model compounds over sulphided NiO-MoO3/γ-Al2O3 and Nio-WO 3/γ-Al2O3 catalysts; chemical evidence for the existence of two types of catalytic sites. Catal Today 4:117–131
Gevert BS, Otterstedt JE, Mssoth FE (1987) Kinetics of the HDO of methyl-substituted phenols. Appl Catal 31:119–131
Furimsky E (2000) Catalytic hydrodeoxygenation. Appl Catal A 199:147–190
Elliott DC, Hart TR, Neuenschwander GG (2009) Catalytic hydroprocessing of biomass fast pyrolysis bio-oil to produce hydrocarbon products. Prog Sustain Energy 28:441–449
Prasomsri T, Shetty M, Murugappan K (2014) Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures. Energy Environ Sci 7:2660–2669
Boullosa-Eiras S, LøDeng R, Bergem H (2014) Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts. Catal Today 223:44–53
Ryymin EM, Honkela ML, Viljava TR (2010) Competitive reactions and mechanisms in the simultaneous HDO of phenol and methyl heptanoate over sulphided NiMo/γ-Al2O3. Appl Catal A 389:114–121
Zhu Y, Zhang Y, Dan Y (2015) Optimization of reaction variables and macrokinetics for the hydrodeoxygenation of full range low temperature coal tar. React Kinet Mech Catal 116:433–450
Girgis MJ, Gates BC (1991) Reactivities, reaction networks, and kinetics in high-pressure catalytic hydroprocessing. Ind Eng Chem Res 30:2021–2058
Song ZY, Yan Y, Niu M (2019) Study on the Process and kinetics of low temperature coal tar hydrodeoxygenation. Chem React Eng Technol 35:3–13
Ni H, Xu C, Wang R (2018) Composition and transformation of sulfur-, oxygen-, and nitrogen-containing compounds in the hydrotreating process of a low-temperature coal tar. Energy Fuels 32:3077–3084
Sun J, Li D, Yao R (2014) Modeling the hydrotreatment of full range medium temperature coal tar by using a lumping kinetic approach. React Kinet Mech Catal 114:451–471
Dan Y, Zhu Y, Li D (2020) Lumped kinetic simulation of hydrodesulfurization of full-range low temperature coal tar. Energy Sources, Part A 6:1–13
Fei D, Gao M, Li C (2011) Detailed description of coal tar hydrogenation process using the kinetic lumping approach. Energy Fuels 25:4878–4885
Tian F, Zhu Y, Liu J (2020) Optimization and lumped kinetic model study of coal-based aerospace kerosene hydrogenation process. React Kinet Mech Catal 130(2):753–775
Faraji D, Zabihi S, Ghadiri M (2020) Computational fluid dynamic modeling and simulation of hydrocracking of vacuum gas oil in a fixed-bed reactor. ACS Omega 5:16595–16601
Fei D, Gao M, Li C (2015) New kinetic model of coal tar hydrogenation process via carbon number component approach. Appl Energy 137:265–272
Xu HS, Zhong F, Xu S (2017) Progress in presulfurization technology of hydrogenation catalyst. Petrochem Technol Appl 3:71–75
Fang X, Fang XC, Cheng Z (2005) Study on technique of refining straight-run distillate diesel oil by ammonia-alcohol method. Contemp Chem Ind 34:145–148
Li D, Li WH, Liu QC (2015) Optimization of processing parameters and macrokinetics for hydrodesulfurization of coal tar. Energy Sources Part A 37:2591–2600
Ternan M, Brown JR (1982) Hydrotreating a distillate liquid derived from subbituminous coal using a sulphided CoO-MoO3Al2O3 catalyst. Fuel 61:1110–1118
Leckel D (2008) Hydrodeoxygenation of Heavy oils derived from low-temperature coal gasification over NiW catalysts—effect of pore structure. Energy Fuels 22:231–236
Krishnamurthy S, Panvelker S, Shah YT (1981) Hydrodeoxygenation of dibenzofuran and related compounds. AIChE J 27:994–1001
Tang X, Li SY, Yue CT (2015) Lumping kinetic models of shale oil hydrodenitrogenation. Acta Pet. Sin Pet Process Sect 31:1354–1362
Acknowledgements
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (21978237), Shaanxi Technology Innovation Shaanxi Key Research and Development Plan Project (2018zdxm-gy-161), Natural Science Basic Research Program of Shaanxi (Program No. 2021JLM-19), Natural Science Basic Research Program of Shaanxi (Program No. 2019JLP-03).
Author information
Authors and Affiliations
Contributions
YW: Writing—review & editing, Methodology, Investigation, Writing—original draft. YZ: Software, Validation. FT: Software. HD: Data curation. XF: Formal analysis. CD: Validation. LC: Supervision, Resources. HZ: Project administration. HT: Project administration, Investigation, Resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhu, Y., Tian, F. et al. Lumped kinetic model for the hydrodeoxygenation of full-range middle-low temperature coal tar. Reac Kinet Mech Cat 134, 179–198 (2021). https://doi.org/10.1007/s11144-021-02053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02053-1