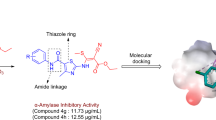
Abstract
The α-amylase is the main product of pancreas and is necessarily involved in the hydrolysis of carbohydrates into glucose so that it has been known to be a pioneer target for type 2 Diabetes mellitus (DM). Type 2 DM has no certain cure and the global increase in the cases of DM requires effective and extensive number of drug candidates. Drug discovery studies using organic biochemistry approaches are of important to describe novel compounds. This study aimed to reveal inhibitory potential of 13 novel compounds containing piperazine or benzimidazole moieties on α-amylase. The novel compounds were synthesized, structurally corroborated by various spectral analysis (FTIR, UV-Vis, 1H NMR and 13C NMR) and screened for anti α-amylase activity. Among the synthesized derivatives, compound 14 was found to be the most potent inhibitor of α-amylase having IC50 64.8 ± 1.8 μM. Inhibition types and Ki values of the most effective molecules (14 and 10a with different moieties) were further investigated. Molecular docking studies were conducted to correlate the outcome of in vitro biochemical kinetic assays and therefore rationalize the binding interactions. In vitro cytotoxicity studies on pancreatic cancer (AR42J) cells were then performed for compound14, and the compound was found to be more effective compared to the positive control, acarbose. Prediction of in silico ADME properties of all tested molecules were determined.









Similar content being viewed by others
Data availability
The datasets generated during/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets supporting the results of this paper are included within the paper and its additional files.
References
Borah PK, Sarkar A, Duary RK. Water-soluble vitamins for controlling starch digestion: Conformational scrambling and inhibition mechanism of human pancreatic α-amylase by ascorbic acid and folic acid. Food Chem. 2019;288:395–404.
Ganesan MS, Kanmani Raja K, Narasimhan K, Murugesan S, Karan Kumar B. Design, synthesis, α-amylase inhibition and in silico docking study of novel quinoline bearing proline derivatives. J Mol Struct. 2020;1208:127873.
Cardullo N, Muccilli V, Pulvirenti L, Cornu A, Pouységu L, Deffieux D, et al. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: a study of α-glucosidase and α-amylase inhibition. Food Chem. 2020;313:126099.
Zheng Y, Tian J, Yang W, Chen S, Liu D, Fang H, et al. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020;317:126346.
Hemlata B, Pornima G, Tukaram K, Pankaj B. In vitro anti-amylase activity of some Indian dietary spices. J Appl Biol Biotechnol. 2019;7:70–4.
Woodford N. Biological counterstrike: antibiotic resistance mechanisms of gram-positive cocci. Clin Microbiol Infect. 2005;23:2–21.
Maruyama T, Kano Y, Yamamoto Y, Kurazono M, Iwamatsu K, Atsumi K, et al. Synthesis and SAR study of novel 7-(pyridinium-3-yl)-carbonyl imidazo[5,1-b]thiazol-2-yl carbapenems. Bioorg Med Chem. 2007;15:392–402.
Demirbas A, Sahin D, Demirbas N, Karaoglu SA. Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur J Med Chem. 2009;44:2896–903.
Barbuceanu SF, Saramet G, Almajan LG, Draghici C, Barbuceanu F, Bancescu G. New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenylsulfone moieties. Synthesis, characterization and antimicrobial activity evaluation. Eur J Med Chem. 2012;49:417–23.
Deng X, Qiu Q, Yang B, Wang X, Huang W, Qian H. Design, synthesis and biological evaluation of novel peptides with anti-cancer and drug resistance-reversing activities. Eur J Med Chem. 2015;89:540–8. 2015
Basoglu S, Ulker S, Alpay-Karaoglu S, Demirbas N. Microwave-assisted synthesis of some hybrid molecules containing penicillanic acid or cephalosporanic acid moieties and investigation of their biological activities. Med Chem Res. 2014;23:3128–43.
Sharma S, Wakode F, Fayaz S, Khasimbi F, Pottoo H, Kaur A. An overview of piperazine scaffold as promising nucleus for different therapeutic targets. Curr Pharm Des. 2020;26:1–13.
Adegboye AA, Khan KM, Salar U, Aboaba SA, Kanwal, Chigurupati S, et al. 2-Aryl benzimidazoles: Synthesis, in vitro α-amylase inhibitory activity, and molecular docking study. Eur J Med Chem. 2018;150:248–60.
Kazimierczuk ZJ, Upcroft A, Upcroft P, Gorska A, Starosciak B, Laudy A. Synthesis, antiprotozoal and antibacterial activity of nitro- and halogeno-substituted benzimidazole derivatives. Acta Biochim Pol. 2002;49:185–95.
Ansari KF, Lal C. Synthesis, physicochemical properties and anti-microbial activity of some new benzimidazole derivatives. Eur J Med Chem. 2009;44:4028–33.
Macalino SJY, Gosu V, Hong S, Choi S. Role of computer-aided drug design in modern drug discovery. Arch Pharm Res. 2015;38:1686–701.
Trott O, Olson AJ. Auto Dock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61.
Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. 2011;7:146–57.
Pagadala NS, Syed K, Tuszynski J. Software for molecular docking: a review. Biophys Rev. 2017;9:91–102.
Ferreira LG, Dos Santos RN, Oliva G, Andricopulo AD. Molecular docking and structure-based drug design strategies. Molecules. 2015;20:13384–421.
Rajanarendar E, Thirupathaiah K, Ramakrishna S, Nagaraju D. A facile and convenient synthesis of novel imidazo[1,2-b] isoxazoles and their Mannich bases as potential biodynamic agents. Chin Chem Lett. 2015;26:1511–3.
Mentese MY, Bayrak H, Uygun Y, Mermer A, Ulker S, Karaoğlu SA, et al. Microwave assisted synthesis of some hybrid molecules derived from norfloxacin and investigation of their biological activities. Eur J Med Chem. 2013;67:230–42.
Balabani A, Hadjipavlou-Litina DJ, Litinas KE, Mainou M, Tsironi CC, Vronteli A. Synthesis and biological evaluation of (2,5-dihydro-1H-pyrrol-1-yl)-2H-chromen-2-ones as free radical scavengers. Eur J Med Chem. 2011;46:5894–901.
Bayrak H, Demirbas A, Demirbas N, Karaoglu SA. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur J Med Chem. 2009;44:4362–6.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25.
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23.
Matsuura Y. A possible mechanism of catalysis involving three essential residues in the enzymes of alpha-amylase family. Biol-Bratisl. 2002;57:21–8.
Gilles C, Astier JP, Marchis-Mouren G, Cambillau C, Payan F. Crystal structure of pig pancreatic α‐amylase isoenzyme II, in complex with the carbohydrate inhibitor acarbose. Eur J Biochem. 1996;238:561–9.
Wang J, Zhao J, Yan Y, Liu D, Wang C, Wang H. Inhibition of glycosidase by ursolic acid: In vitro, in vivo and in silico study. J Sci Food Agric. 2020;100:986–94.
Mohammadi-Khanaposhtani M, Rezaei S, Khalifeh R, Imanparast S, Faramarzi MA, Bahadorikhalili S, et al. Design, synthesis, docking study, alpha-glucosidase inhibition, and cytotoxic activities of acridine linked to thioacetamides as novel agents in treatment of type 2 diabetes. Bioorg Chem. 2018;80:288–95.
Yadav V, Talwar P. Repositioning of fluoroquinolones from antibiotic to anti-cancer agents: an underestimated truth. Biomed. 2019;111:934.
Idowu T, Schweizer F. Ubiquitous nature of fluoroquinolones: the oscillation between antibacterial and anticancer activities. Antibiotics. 2017;6:26.
Nishi K, Kato M, Sakurai S, Matsumoto A, Iwase Y, Yumita N. Enoxacin with UVA irradiation induces apoptosis in the AsPC1 human pancreatic cancer cell line through ROS generation. Anticancer Res. 2017;37:6211.
Beberok A, Wrześniok D, Rok J, Rzepka Z, Respondek M, Buszman E. Ciprofloxacin triggers the apoptosis of human triple-negative breast cancer MDAMB- 231 cells via the p53/Bax/Bcl-2 signaling pathway. Int J Oncol. 2018;52:1727–37.
Beberok A, Wrześniok D, Minecka A, Rok J, Delijewski M, Rzepka Z, et al. Ciprofloxacin-mediated induction of S-phase cell cycle arrest and apoptosis in COLO829 melanoma cells. Pharmacol Rep. 2018;70:6.
Yu M, Li R, Zhang J. Repositioning of antibiotic levofloxacin as a mitochondrial biogenesis inhibitor to target breast cancer. Biochem Biophys Res Commun. 2016;471:639–45.
Song M, Wu H, Wu S, Ge T, Wang G, Zhou Y, et al. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed Pharm Ther. 2016;84:1137.
Jemel-Oualha I, Elloumi-Mseddi J, Beji A, Hakim B, Aifa S. Controversial effect on Erk activation of some cytotoxic drugs in human LOVO colon cancer cells. J Recept Transduct Res. 2016;36:21.
Sousa E, Graça I, Baptista T, Vieira FQ, Palmeira C, Henrique R, et al. Enoxacin inhibits growth of prostate cancer cells and effectively restores microRNA processing. Epigenetics. 2013;8:548.
Azema J, Guidetti B, Korolyov A, Kiss R, Roques C, Constant P, et al. Synthesis of lipophilic dimeric C-7/C-7-linked ciprofloxacin and C-6/C-6-linked levofloxacin derivatives. Versatile in vitro biological evaluations of monomeric and dimeric fluoroquinolone derivatives as potential antitumor, antibacterial or antimycobacterial agents. Eur J Med Chem. 2011;46:6025.
Hu G, Wang G, Duan N, Wen X, Cao T, Xie S, et al. Design, synthesis and antitumor activities of fluoroquinolone C-3 heterocycles (IV): s-triazole Schiff–Mannich bases derived from ofloxacin. Acta Pharm Sin B. 2012;2:312.
Dixit S, Mishra N, Sharma M, Singh S, Agarwal A, Awassthi SK, et al. Synthesis and in vitro antiplasmodial activities of fluoroquinolone analogs. Eur J Med Chem. 2012;51:52.
Gürbay A, Osman M, Favier A, Hincal F. Ciprofloxacin-Induced Cytotoxicity and Apoptosis in HeLa Cells. Toxicol Mech Methods. 2005;15:339.
Basoglu-Ozdemir S. Synthesis of novel fluoroquinolone-triazole hybrid compounds as antimicrobial agents. J Turkish Chem Soc Sect Chem. 2016;3:515–34.
Menteşe M, Demirbaş N, Mermer A, Demirci S, Demirbaş A, Ayaz FA. Novel azole-functionalited flouroquinolone hybrids: design, conventional and microwave ırradiated synthesis, evaluation as antibacterial and antioxidant agents. Lett Drug Des Discov. 2017;15:46–64.
Taha M, Baharudin MS, Ismail NH, Imran S, Khan MN, Rahim F, et al. Synthesis, α-amylase inhibitory potential and molecular docking study of indole derivatives. Bioorg Chem. 2018;80:36–42.
Kolcuoglu Y, Colak A, Faiz O, Belduz AO. Cloning, expression and characterization of highly thermo- and pH-stable maltogenic amylase from a thermophilic bacterium Geobacillus caldoxylosilyticus TK4. Proc Biochem. 2010;45:821–8.
Lineweaver H, Burk D. The determination of enzyme dissociation constant. J Am Chem Soc. 1934;56:658–61.
Lv X, Bai R, Yan J-K, Huang H-L, Huo X-K, Tian X-G, et al. Investigation of the inhibitory effect of protostanes on human carboxylesterase 2 and their interaction: inhibition kinetics and molecular stimulations. Int J Biol Macromol. 2021;167:1262–72.
Sun C-P, Yan J-K, Yi J, Zhang X-Y, Yu Z-L, Huo X-K, et al. The study of inhibitory effect of natural flavonoids toward β-glucuronidase and interaction off lavonoids with β-glucuronidase. Int J Biol Macromol. 2020;143:349–58.
Zhao W-Y, Yan J-J, Zhang M, Wang C, Feng L, Lv X, et al. Natural soluble epoxide hydrolase inhibitors from Inula britanica and their potential interactions with soluble epoxide hydrolase: Insight from inhibition kinetics and molecular Dynamics. Chem Biol Interact. 2021;345:109571.
Molinspiration Cheminformatics, http://www.molinspiration.com/cgibin/properties.
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7.1:1–13.
Zhao YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, et al. Rate-limited steps of human oral absorption and QSAR studies. Pharm Res. 2002;19:1446–57.
Spartan’16 Wavefunction, Inc. Irvine, CA.
Stewart JJP. Application of the pm6 method to modeling proteins. J Mol Model. 2009;15:765–805.
Zhao Y, Truhlar DG. Density functionals with broad applicability in chemistry. Acc Chem Res. 2008;41:157–67.
Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc. 2008;120:215–41.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;16:2785–91.
Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–7.
Grosdidier A, Zoete V, Michielin O. Fast docking using the CHARMM force field with EADock DSS. J Comput Chem. 2011;32:2149–59.
BIOVIA, D. S. Discovery Studio Modeling Environment, Release 4.5, San Diego: Dassault Systèmes, 2015.
Demir S, Aliyazicioglu Y, Turan I, Misir S, Mentese A, Ozer-Yaman S, et al. Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line. Nutr Cancer. 2016;68:165–72.
Demir S, Turan I, Aliyazicioglu Y, Kilinc K, Ozer Yaman S, Ayazoglu Demir E, et al. Morus rubra extract induces cell cycle arrest and apoptosis in human colon cancer cells through endoplasmic reticulum stress and telomerase. Nutr Cancer. 2017;69:74.
Eichler HG, Korn A, Gasic S, Pirson W, Businger J. The effect of a new specific alpha-amylase inhibitor on post-prandial glucose and insulin excursions in normal subjects and type-2 (non-insulin-dependent) diabetic-patients. Diabetologia. 1984;26:278–81.
Creutzfeldt W. The [pre-] history of the incretin concept. Regulatory Pept. 2005;128:87–91.
Ayazoglu-Demir E, Demir S, Aliyazıcıoglu Y. In vitro cytotoxic effect of ethanol and dimethyl sulfoxide on various human cell lines. KSU J Agric Nat Scope Focus. 2020;23:1119–24.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Wang HM, Chen CY, Chen CY, Ho ML, Chou YT, Chang HC, et al. (-)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorg Med Chem. 2010;18:5241–7.
Author information
Authors and Affiliations
Contributions
UC: Methodology, Formal analysis, Investigation, Writing—Original draft, Writing—Review & Editing, Visualization, Data curation. FOT: Methodology, Formal analysis, Investigation, Writing—Original draft. SBO: Methodology, Formal analysis, Investigation, Writing—Original draft, Visualization, Data curation. EA-D: Formal analysis, Investigation, Writing—Original draft. ID: Methodology, Software, Formal analysis, Investigation, Writing—Original Draft, Visualization. AC: Conceptualization, Resources, Supervision, Writing—Original draft, Writing—Review & Editing Project administration, Funding acquisition. SC-U: Methodology, Resources. SSE: Methodology, Software, Resources. NY: Formal analysis, Resources.
Funding
The authors are grateful to the Research Fund of the TUBITAK (The Scientific and Technological Research Council of Turkey) or this support with Project No 117Z199.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
All authors have their consent to participate
Consent to publish
The participants gave their consent for publication
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cakmak, U., Oz-Tuncay, F., Basoglu-Ozdemir, S. et al. Synthesis of hydrazine containing piperazine or benzimidazole derivatives and their potential as α-amylase inhibitors by molecular docking, inhibition kinetics and in vitro cytotoxicity activity studies. Med Chem Res 30, 1886–1904 (2021). https://doi.org/10.1007/s00044-021-02785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02785-8