Abstract
Purpose
To investigate the efficacy of sequential apatinib treatment in patients with unresectable hepatocellular carcinoma (HCC) refractory to sorafenib–transarterial chemoembolization (TACE).
Materials and methods
This respective study was conducted on 95 consecutive patients with sorafenib–TACE-refractory unresectable HCC in our center from January 2014 to December 2019. According to the response to sorafenib–TACE treatment and the subsequent management, the eligible patients were assigned into three groups: sorafenib–TACE (ST) group, sorafenib–TACE–apatinib (STA) group, and sorafenib–TACE–supportive care (STS) group. The differences in overall survival (OS) and tumor response were compared among the three groups. Risk factors related to prognosis were analyzed.
Results
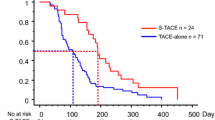
A total of 58 patients were enrolled in the study, with 11, 15, and 28 patients in ST, STA, and STS group, respectively. The median OS of the STA group was significantly improved when compared with the STS group, either from the start of sorafenib–TACE resistance or the initiation of sorafenib–TACE therapy (14.0 versus 4.0 months, p = 0.003; 19.0 versus 9.0 months, p < 0.001; respectively). Additionally, from the start of sorafenib–TACE treatment, the median OS of the STA group was longer than that of the ST group without statistical difference (19.0 versus 15.0 months, p = 0.28), so did the comparison of median OS between the ST group and the STS group (15.0 versus 9.0 months, p = 0.06). Extrahepatic metastases were an independent risk factor for poor prognosis.
Conclusion
In patients with sorafenib–TACE-refractory HCC, subsequent apatinib treatment significantly improved the OS when compared with supportive care, and produced comparative outcomes with those sorafenib–TACE responders.



Similar content being viewed by others
References
Sung H, Ferlay, J, Siegel, RL et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020. https://doi.org/10.3322/caac.21660.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56: 908–43.
Cheng A-L, Kang Y-K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66.
Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–70.
Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–80.
Qin S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: a phase II randomized, open-label trial. J Clin Oncol. 2014;32:4019–4019.
Zhen L, Jiali C, Yong F, et al. The efficacy and safety of apatinib treatment for patients with unresectable or relapsed liver cancer: a retrospective study. J Cancer. 2018;9:2773–7.
Kan X, Liang B, Zhou G, et al. Transarterial chemoembolization combined with apatinib for advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. 2020;10:970.
Zhang Y, Huang G, Miao H, et al. Apatinib treatment may improve survival outcomes of patients with hepatitis B virus-related sorafenib-resistant hepatocellular carcinoma. Ther Adv Med Oncol. 2020;12:1758835920937422.
Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–501.
Zhang Y, Fan W, Wang Y, et al. Apatinib for Patients with sorafenib-refractory advanced hepatitis B virus related hepatocellular carcinoma: results of a pilot study. Cancer Control. 2019;26:1073274819872216.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31.
Palmer DH. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498; author reply 2498–9.
Kim HY, Park JW, Joo J, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1051–6.
Arizumi T, Minami T, Chishina H, et al. Time to Transcatheter arterial chemoembolization refractoriness in patients with hepatocellular carcinoma in kinki criteria stages B1 and B2. Dig Dis. 2017;35:589–97.
Liu KC, Hao YH, Lv WF, et al. Transarterial Chemoembolization combined with sorafenib in patients with BCLC stage C hepatocellular carcinoma. Drug Des Dev Ther. 2020;14:3461–8.
Yuan J, Yin X, Tang B, et al. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Biomed Res Int. 2019;2019:2141859.
Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353–8.
Liu K, Min XL, Peng J, et al. The changes of HIF-1alpha and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8:297–302.
Shim JH, Park J-W, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–44.
Niu L, Liu L, Yang S, et al. New insights into sorafenib resistance in hepatocellular carcinoma: responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer. 2017;1868:564–70.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 81873919).
Author information
Authors and Affiliations
Contributions
Chuansheng Zheng conceived and designed the study. Yanyan Cao contributed significantly to analysis and manuscript preparation. Tao Ouyang performed the data analysis and wrote the manuscript. Fu Xiong, Xuefeng Kan, Lei Chen, and Bin Liang helped to perform the analysis with constructive discussions. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The study was conducted according to the Declaration of Helsinki. Informed consent was waived for the retrospective type of this study. The information of all participants is maintained with confidentiality. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent to participates
All patients were informed that apatinib was an alternative treatment, because the first-line sorafenib–TACE treatment was ineffective. Moreover, all patients were informed of the economic cost, anticipated outcomes, and potential toxicity. Final treatment decisions were made by the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, Y., Ouyang, T., Xiong, F. et al. Efficacy of apatinib in patients with sorafenib–transarterial chemoembolization refractory hepatocellular carcinoma: a retrospective study. Hepatol Int 15, 1268–1277 (2021). https://doi.org/10.1007/s12072-021-10198-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10198-3