Abstract
Two novel unsymmetrical binucleating aroylhydrazonic ligands and four dicopper(II) complexes carrying fluorescent benzopyranothiophene (BPT) or boron dipyrromethene (BODIPY) entities were synthesized and fully characterized. Complex 1, derived from the BPT-containing ligand H3L1, had its crystal structure elucidated through X-ray diffraction measurements. The absorption and fluorescence profiles of all the compounds obtained were discussed. Additionally, the stability of the ligands and complexes was monitored by UV–vis spectroscopy in DMSO and biologically relevant media. All the compounds showed moderate to high cytotoxicity towards the triple negative human breast cancer cell line MDA-MB-231. BPT derivatives were the most cytotoxic, specially H3L1, reaching an IC50 value up to the nanomolar range. Finally, fluorescence microscopy imaging studies employing mitochondria- and nucleus-staining dyes showed that the BODIPY-carrying ligand H3L2 was highly cell permeant and suggested that the compound preferentially accumulates in the mitochondria.
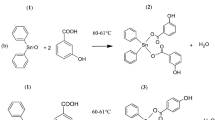
Graphic abstract








Similar content being viewed by others
References
Festa RA, Thiele DJ (2011) Copper: an essential metal in biology. Curr Biol 21:R877-883. https://doi.org/10.1016/j.cub.2011.09.040
Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L (2014) Copper active sites in biology. Chem Rev 114:3659–3853. https://doi.org/10.1021/cr400327t
Erxleben A (2018) Interactions of copper complexes with nucleic acids. Coord Chem Rev 360:92–121. https://doi.org/10.1016/j.ccr.2018.01.008
McGivern TJP, Afsharpour S, Marmion CJ (2018) Copper complexes as artificial DNA metallonucleases: from Sigman’s reagent to next generation anti-cancer agent? Inorg Chim Acta 472:12–39. https://doi.org/10.1016/j.ica.2017.08.043
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Advances in copper complexes as anticancer agents. Chem Rev 114:815–862. https://doi.org/10.1021/cr400135x
Ainscough EW, Brodie AM, Denny WA, Finlay GJ, Gothe SA, Ranford JD (1999) Cytotoxicity of salicylaldehyde benzoylhydrazone analogs and their transition metal complexes: quantitative structure-activity relationships. J Inorg Biochem 77:125–133. https://doi.org/10.1016/s0162-0134(99)00131-2
Lee WY, Lee PP, Yan YK, Lau M (2010) Cytotoxic copper(II) salicylaldehyde semicarbazone complexes: mode of action and proteomic analysis. Metallomics 2:694–705. https://doi.org/10.1039/c0mt00016g
Raja DS, Bhuvanesh NSP, Natarajan K (2012) DNA binding, protein interaction, radical scavenging and cytotoxic activity of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde(2′-hydroxybenzoyl)hydrazone and its Cu(II) complexes: a structure activity relationship study. Inorg Chim Acta 385:81–93. https://doi.org/10.1016/j.ica.2011.12.038
Cindrić M, Bjelopetrović A, Pavlović G, Damjanović V, Lovrić J, Matković-Čalogović D, Vrdoljak V (2017) Copper(II) complexes with benzhydrazone-related ligands: synthesis, structural studies and cytotoxicity assay. New J Chem 41:2425–2435. https://doi.org/10.1039/C6NJ03827A
Ramachandran E, Gandin V, Bertani R, Sgarbossa P, Natarajan K, Bhuvanesh NSP, Venzo A, Zoleo A, Glisenti A, Dolmella A, Albinati A, Marzano C (2018) Synthesis, characterization and cytotoxic activity of novel copper(II) complexes with aroylhydrazone derivatives of 2-Oxo-1,2-dihydrobenzo[h]quinoline-3-carbaldehyde. J Inorg Biochem 182:18–28. https://doi.org/10.1016/j.jinorgbio.2018.01.016
Burgos-Lopez Y, Del Plá J, Balsa LM, León IE, Echeverría GA, Piro OE, García-Tojal J, Pis-Diez R, González-Baró AC, Parajón-Costa BS (2019) Synthesis, crystal structure and cytotoxicity assays of a copper(II) nitrate complex with a tridentate ONO acylhydrazone ligand. Spectroscopic and theoretical studies of the complex and its ligand. Inorg Chim Acta 487:31–40. https://doi.org/10.1016/j.ica.2018.11.039
Ji Y, Dai F, Zhou B (2018) Designing salicylaldehyde isonicotinoyl hydrazones as Cu(II) ionophores with tunable chelation and release of copper for hitting redox Achilles heel of cancer cells. Free Radic Biol Med 129:215–226. https://doi.org/10.1016/j.freeradbiomed.2018.09.017
Rey NA, Neves A, Bortoluzzi AJ, Haase W, Tomkowicz Z (2012) Doubly phenoxo–hydroxo-bridged dicopper(II) complexes: individual contributions of the bridges to antiferromagnetic coupling based on two related biomimetic models for catechol oxidases. Dalton Trans 41:7196–7200. https://doi.org/10.1039/C2DT30419H
Rey NA, Neves A, Bortoluzzi AJ, Pich CT, Terenzi H (2007) Catalytic promiscuity in biomimetic systems: catecholase-like activity, phosphatase-like activity, and hydrolytic DNA cleavage promoted by a new dicopper(II) hydroxo-bridged complex. Inorg Chem 46:348–350. https://doi.org/10.1021/ic0613107
Rada JP, Bastos BSM, Anselmino L, Franco CHJ, Lanznaster M, Diniz R, Fernández CO, Menacho-Márquez M, Percebom AM, Rey NA (2019) Binucleating hydrazonic ligands and their μ-hydroxodicopper(II) complexes as promising structural motifs for enhanced antitumor activity. Inorg Chem 58:8800–8819. https://doi.org/10.1021/acs.inorgchem.9b01195
Rada JP, Forté J, Gontard G, Corcé V, Salmain M, Rey NA (2020) Isoxazole-derived aroylhydrazones and their dinuclear copper(II) complexes show antiproliferative activity on breast cancer cells with a potentially alternative mechanism of action. ChemBioChem 21:2474–2486. https://doi.org/10.1002/cbic.202000122
Bertrand B, Passador K, Goze C, Denat F, Bodio E, Salmain M (2018) Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord Chem Rev 358:108–124. https://doi.org/10.1016/j.ccr.2017.12.007
Bhattacharyya A, Jameei A, Garai A, Saha R, Karande AA, Chakravarty AR (2018) Mitochondria-localizing BODIPY–copper(II) conjugates for cellular imaging and photo-activated cytotoxicity forming singlet oxygen. Dalton Trans 47:5019–5030. https://doi.org/10.1039/C8DT00255J
Bhattacharyya A, Dixit A, Mitra K, Banerjee S, Karande AA, Chakravarty AR (2015) BODIPY appended copper(ii) complexes of curcumin showing mitochondria targeted remarkable photocytotoxicity in visible light. MedChemComm 6:846–851. https://doi.org/10.1039/C4MD00425F
Bhattacharyya A, Dixit A, Banerjee S, Roy B, Kumar A, Karande AA, Chakravarty AR (2016) BODIPY appended copper(ii) complexes for cellular imaging and singlet oxygen mediated anticancer activity in visible light. RSC Adv 6:104474–104482. https://doi.org/10.1039/C6RA23118G
Mukherjee N, Podder S, Mitra K, Majumdar S, Nandi D, Chakravarty AR (2018) Targeted photodynamic therapy in visible light using BODIPY-appended copper(II) complexes of a vitamin B6 Schiff base. Dalton Trans 47:823–835. https://doi.org/10.1039/C7DT03976J
Zhu Z, Zhou X, Wang Y, Chi L, Ruan D, Xuan Y, Cong W, Jin L (2014) Fluorescent staining of glycoproteins in sodium dodecyl sulfate polyacrylamide gels by 4H-[1]-benzopyrano[4,3-b]thiophene-2-carboxylic acid hydrazide. Analyst 139:2764–2773. https://doi.org/10.1039/C3AN02309E
Jovito R, Neves A, Bortoluzzi AJ, Lanznaster M, Drago V, Haase W (2005) A new unsymmetrical dinucleating ligand and its first FeIIIZnII complex: structure and solid state properties of an unexpected tetranuclear complex containing the [FeIII(μ-OH)2FeIII] structural motif. Inorg Chem Commun 8:323–327. https://doi.org/10.1016/j.inoche.2005.01.007
Palatinus L, Chapuis G (2007) SUPERFLIP—a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Crystallogr 40:786–790. https://doi.org/10.1107/S0021889807029238
Sheldrick G (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C 71:3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Qin W, Baruah M, De Borggraeve WM, Boens N (2006) Photophysical properties of an on/off fluorescent pH indicator excitable with visible light based on a borondipyrromethene-linked phenol. J Photochem Photobiol A 183:190–197. https://doi.org/10.1016/j.jphotochem.2006.03.015
Ashokkumar P, Weißhoff H, Kraus W, Rurack K (2014) Test-strip-based fluorometric detection of fluoride in aqueous media with a BODIPY-linked hydrogen-bonding receptor. Angew Chem Int Ed 53:2225–2229. https://doi.org/10.1002/anie.201307848
Palla G, Predieri G, Domiano P, Vignali C, Turner W (1986) Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron 42:3649–3654. https://doi.org/10.1016/S0040-4020(01)87332-4
Shen B-X, Qian Y, Qi Z-Q, Lu C-G, Sun Q, Xia X, Cui Y-P (2017) Near-infrared BODIPY-based two-photon ClO− probe based on thiosemicarbazide desulfurization reaction: naked-eye detection and mitochondrial imaging. J Mater Chem B 5:5854–5861. https://doi.org/10.1039/C7TB01344B
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122. https://doi.org/10.1016/S0010-8545(00)80009-0
Lavanant H, Virelizier H, Hoppilliard Y (1998) Reduction of copper(II) complexes by electron capture in an electrospray ionization source. J Am Soc Mass Spectrom 9:1217–1221. https://doi.org/10.1021/jasms.8b01118
Reis ACDM, Freitas MCR, Resende JALC, Diniz R, Rey NA (2014) Different coordination patterns for two related unsymmetrical compartmental ligands: crystal structures and IR analysis of [Cu(C21H21O2N3)(OH2)(ClO4)]ClO4·2H2O and [Zn2(C22H21O3N2)(C22H20O3N2)]ClO4. J Coord Chem 67:3067–3083. https://doi.org/10.1080/00958972.2014.958080
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4932. https://doi.org/10.1021/cr078381n
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed Engl 47:1184–1201. https://doi.org/10.1002/anie.200702070
Castillo M, Raut SL, Price S, Bora I, Jameson LP, Qiu C, Schug KA, Gryczynski Z, Dzyuba SV (2016) Spectroscopic differentiation between monomeric and aggregated forms of BODIPY dyes: effect of 1,1-dichloroethane. RSC Adv 6:68705–68708. https://doi.org/10.1039/C6RA10833D
Giovagnini L, Sitran S, Montopoli M, Caparrotta L, Corsini M, Rosani C, Zanello P, Dou QP, Fregona D (2008) Chemical and biological profiles of novel copper(II) complexes containing S-donor ligands for the treatment of cancer. Inorg Chem 47(14):6336–6343
Acknowledgements
Nicolás A. Rey thanks FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) for the research fellowships awarded. J. P. Rada appreciates the financial support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil) through the fellowship PDSE-88881.187965/2018-0 and the scholarship 88887.151652/2017-00, as well as CNPq (GM/GD-140228/2018-7). In addition, Prof. Ricardo Q. Aucélio (Department of Chemistry, PUC-Rio) is gratefully acknowledged for the elemental analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rada, J.P., Forté, J., Gontard, G. et al. Novel luminescent benzopyranothiophene- and BODIPY-derived aroylhydrazonic ligands and their dicopper(II) complexes: syntheses, antiproliferative activity and cellular uptake studies. J Biol Inorg Chem 26, 675–688 (2021). https://doi.org/10.1007/s00775-021-01885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01885-5