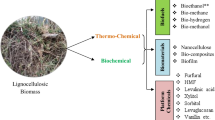
Abstract
The use of lignocellulosic biomass (LCB) has emerged as one of the main strategies for generating renewable biofuels. For the efficient use of such feedstock, pre-treatments are essential. The hydrolysis of cellulose – major component of LCB – demands enzymatic cocktails with improved efficiency to generate fermentable sugars. In this scenario, lignocellulolytic fungi have enormous potential for the development of efficient enzyme platforms. In this study, two enzymatic cocktails were developed for hydrolysis of two lignocellulosic biomasses: industrial cellulose pulp and cassava peel. The solid biomass ratio in relation to the protein content of the enzyme cocktail was performed by experimental design. The optimized cocktail for the hydrolysis of cellulose pulp (AMZ 1) was composed, in protein base, by 43% of Aspergillus sp. LMI03 enzyme extract and 57% of T. reesei QM9414, while the optimal enzyme cocktail for cassava peel hydrolysis (AMZ 2) was composed by 50% of Aspergillus sp. LMI03 enzyme extract, 25% of the extract of P. citrinum LMI01 and 25% of T. reesei. The ratio between solids and protein loading for AMZ 1 cocktail performance was 52 g/L solids and 30 mg protein/g solids, resulting in a hydrolytic efficiency of 93%. For the AMZ 2 cocktail, the hydrolytic efficiency was 78% for an optimized ratio of 78 g/L solids and 19 mg protein/g solids. These results indicate that cocktails formulated with enzymatic extracts of P. citrinum LMI01, Aspergillus sp. LMI03, and T. reesei QM9414 are excellent alternatives for efficient hydrolysis of plant biomass and for other processes that depend on biocatalysis.






Similar content being viewed by others
References
Pereira Jr, N., Couto, M. A. P. G., & Santa Anna, L. M. M. (2008). Biomass of lignocellulosic composition for fuel ethanol production within the context of biorefinery. Series on Biotechnology (Vol. 2).
Adsul, M., Sandhu, S. K., Singhania, R. R., Gupta, R., Puri, S. K., & Mathur, A. (2020). Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzyme and Microbial Technology, 133, 109442. https://doi.org/10.1016/j.enzmictec.2019.109442
Mandels, M., & Reese, E. (1957). Induction of Cellulase in Trichoderma Viride As Influenced By Carbon Sources and Metals. Journal of Bacteriology, 73(2), 269–278. https://doi.org/10.1128/jb.73.2.269-278.1957
Bischof, R. H., & Ramoni, J. (2016). Seiboth B 2016 Cellulases and beyond: The first 70Â years of the enzyme producer Trichoderma reesei. Microbial Cell Factories, 15(1), 106. https://doi.org/10.1186/S12934-016-0507-6
Dashtban, M., Buchkowski, R., & Qin, W. (2011). Effect of different carbon sources on cellulase production by Hypocrea jecorina (Trichoderma reesei) strains. International Journal of Biochemistry and Molecular Biology, 2(3), 274–86. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3193291/pdf/ijbmb0002-0274.pdf. Accessed 05.28.2019.
Zabed, H., Sahu, J. N., Boyce, A. N., & Faruq, G. (2016). Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renewable and Sustainable Energy Reviews, 66, 751–774. https://doi.org/10.1016/j.rser.2016.08.038
Sun, F. F., Hong, J., Hu, J., Saddler, J. N., Fang, X., Zhang, Z., & Shen, S. (2015). Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme and Microbial Technology, 79–80, 42–48. https://doi.org/10.1016/j.enzmictec.2015.06.020
Valadares, F., Gonçalves, T. A., Gonçalves, D. S. P. O., Segato, F., Romanel, E., Milagres, A. M. F., & Ferraz, A. (2016). Exploring glycoside hydrolases and accessory proteins from wood decay fungi to enhance sugarcane bagasse saccharification. Biotechnology for Biofuels, 9(1), 110. https://doi.org/10.1186/s13068-016-0525-y
Levasseur, A., Drula, E., Lombard, V., Coutinho, P. M., & Henrissat, B. (2013). Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnology for Biofuels, 6(1), 41. https://doi.org/10.1186/1754-6834-6-41
Eijsink, V. G. H., Ludwicka, K., Felice, A. K. G., Preims, M., Ludwig, R., Breslmayr, E., & Scheiblbrandner, S. (2016). Extracellular electron transfer systems fuel cellulose oxidative degradation. Science, 352(6289), 1098-1101.https://doi.org/10.1126/science.aaf3165
Saloheimo, M., Paloheimo, M., Hakola, S., Pere, J., Swanson, B., & Nyysso, E. (2002). Swollenin, a Trichoderma reeseiprotein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. European journal of biochemistry / FEBS, 4211, 4202–4211. https://doi.org/10.1046/j.1432-1033.2002.03095.x
Obeng, E. M., Adam, S. N. N., Budiman, C., Ongkudon, C. M., Maas, R., & Jose, J. (2017). Lignocellulases: A review of emerging and developing enzymes, systems, and practices. Bioresources and Bioprocessing, 4(1). https://doi.org/10.1186/s40643-017-0146-8
Cesar, F., Maria, B., Silvello, A., & Goldbeck, R. (2020). Cellulase and oxidative enzymes : New approaches, challenges and perspectives on cellulose degradation for bioethanol production. Biotechnology Letters, 42(6), 875–884. https://doi.org/10.1007/s10529-020-02875-4
Mohanram, S., Amat, D., Choudhary, J., Arora, A., & Nain, L. (2013). Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustainable Chemical Processes, 1(1), 15. https://doi.org/10.1186/2043-7129-1-15
Borin, G. P., Sanchez, C. C., de Santana, E. S., Zanini, G. K., dos Santos, R. A. C., de Oliveira Pontes, A., & Oliveira, J. V. de C. (2017). Comparative transcriptome analysis reveals different strategies for degradation of steam-exploded sugarcane bagasse by Aspergillus niger and Trichoderma reesei. BMC Genomics, 18(1), 501. https://doi.org/10.1186/s12864-017-3857-5
Lynd, L. R., Weimer, P. J., Zyl, W. H. Van, Isak, S., & Pretorius, I. S. (2002). Microbial cellulose utilization : Fundamentals and biotechnology microbial cellulose utilization . Fundamentals and Biotechnology, 66(3). https://doi.org/10.1128/MMBR.66.3.506
Schuster, A., Schmoll, M., Martins, F. L., Kolling, D., Camassola, M., Jose, A., & Singh, O. V. (2010). Biology and biotechnology of Trichoderma. Journal of Industrial Microbiology & Biotechnology, 87(3), 787-799.https://doi.org/10.1016/S0960-8524(03)00195-0
Seiboth, B., Ivanova, C., & Seidl-seiboth, V. (2011). Trichoderma reesei: A Fungal Enzyme Producer for Cellulosic Biofuels. In Biofuel Production-Recent Developments and Prospects. InTech. https://doi.org/10.5772/16848
Singhania, R. R., Patel, A. K., Sukumaran, R. K., Larroche, C., & Pandey, A. (2013). Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresource Technology, 127, 500–507. https://doi.org/10.1016/j.biortech.2012.09.012
Santa-Rosa, P. S., Souza, A. L., Roque, R. A., Andrade, E. V., Astolfi-Filho, S., Mota, A. J., & Nunes-Silva, C. G. (2018). Production of thermostable β-glucosidase and CMCase by Penicillium sp. LMI01 isolated from the Amazon region. Electronic Journal of Biotechnology, 31, 84–92. https://doi.org/10.1016/j.ejbt.2017.11.005
Mandels, M., & Weber, J. (1969). The production of cellulases. Advances in Chemistry, 95, 391–414. https://doi.org/10.1021/ba-1969-0095
Laemmli, U. K. (1970). Cleavage of Structural Proteins during Assembly of Head of Bacteriophage‐T4. Nature, 227, 680–685. https://doi.org/10.1038/227680a0
Schwarz, W. H., Bronnenmeier, K., Staudenbauer, W. L. (1987). Activity staining of cellulases in polyacrylamide gels containing mixed linkage β-glucans. Analytical Biochemistry, 164(1), 72–77. https://doi.org/10.1016/0003-2697(87)90369-1
Barcelos, C. A., Maeda, R. N., Betancur, G. J. V., & Pereira, N. (2013). The essentialness of delignification on enzymatic hydrolysis of sugar cane bagasse cellulignin for second generation ethanol production. Waste and Biomass Valorization, 4(2), 341–346. https://doi.org/10.1007/s12649-012-9137-3
Ghose, T. K. (1987). Measurement of cellulase activities. Pure & Applied Chemical, 59(2), 257–268.
Eveleigh, D. E., Mandels, M., Andreotti, R., & Roche, C. (2009). Measurement of saccharifying cellulase. Biotechnology for Biofuels, 2(1), 21. https://doi.org/10.1186/1754-6834-2-21
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Li, J., Zhou, P., Liu, H., Xiong, C., Lin, J., Xiao, W., & Liu, Z. (2014). Synergism of cellulase, xylanase, and pectinase on hydrolyzing sugarcane bagasse resulting from different pretreatment technologies. Bioresource Technology, 155, 258–265. https://doi.org/10.1016/j.biortech.2013.12.113
Zerva, A., Pentari, C., Grisel, S., Berrin, J. G., & Topakas, E. (2020). Biotechnology for biofuels a new synergistic relationship between xylan - active LPMO and xylobiohydrolase to tackle recalcitrant xylan. Biotechnology for Biofuels, 1–13. https://doi.org/10.1186/s13068-020-01777-x
Marjamaa, K., Toth, K., Bromann, P. A., Szakacs, G., & Kruus, K. (2013). Novel Penicillium cellulases for total hydrolysis of lignocellulosics. Enzyme and Microbial Technology, 52(6–7), 358–369. https://doi.org/10.1016/j.enzmictec.2013.03.003
Rocha, V. A. L., Maeda, R. N., Santa Anna, L. M. M., & Pereira, N., Jr. (2013). Sugarcane bagasse as feedstock for cellulase production by Trichoderma harzianum in optimized culture medium. Journal of BElehnologyctroniciotec, 16(5), 1–13. https://doi.org/10.2225/vol16-issue5-fulltext-1
Nathan, V., Esther Rani, M., Rathinasamy, G., Dhiraviam, K., & Jayavel, S. (2014). Process optimization and production kinetics for cellulase production by Trichoderma viride VKF3. Springerplus, 3(1), 92. https://doi.org/10.1186/2193-1801-3-92
Benoliel, B., Torres, F. A. G., & de Moraes, L. M. P. (2013). A novel promising Trichoderma harzianum strain for the production of a cellulolytic complex using sugarcane bagasse in natura. Springerplus, 2, 656. https://doi.org/10.1186/2193-1801-2-656
Pirzadah, T., Garg, S., Singh, J., Vyas, A., Kumar, M., Gaur, N., & Bala, M. (2014). Characterization of actinomycetes and Trichoderma spp. for cellulase production utilizing crude substrates by response surface methodology, SpringerPlus, 3(622), 1–12. https://doi.org/10.1186/2193-1801-3-622
Reyes-Sosa, F. M., López Morales, M., Platero Gómez, A. I., Valbuena Crespo, N., Sánchez Zamorano, L., Rocha-Martín J., & DíezGarcía, B. (2017). Management of enzyme diversity in high-performance cellulolytic cocktails. Biotechnology for Biofuels, 10(1), 1–10.https://doi.org/10.1186/s13068-017-0845-6
de Mojana di Cologna, N., Gómez-Mendoza, D. P., Zanoelo, F. F., Giannesi, G. C., de Alencar Guimarães, N. C., de Souza Moreira, L. R., & Ricart, C. A. O. (2018).Exploring Trichoderma and Aspergillus secretomes: Proteomics approaches for the identification of enzymes of biotechnological interest. Enzyme and Microbial Technology, 109, 1–10. https://doi.org/10.1016/J.ENZMICTEC.2017.08.007
Sajith, S., Sreedevi, S., Priji, P., Unni, K. N., & Benjamin, S. (2015). Production and partial purification of cellulase from a new isolate, Penicillium verruculosum BS3. British Microbiology Research Journal, 9(1), 1–12. https://doi.org/10.9734/BMRJ/2015/17865
Parambath, J. N., Valsala, G., & Krishnan, S. R. (2016). Purification and characterization of carboxymethyl cellulase ( CMCase ) from P enicillium ochrochloron isolated from forest soil of Neyyar Wild Life Sanctuary, India. International Journal of Biotechnology and Biochemistry, 12(2), 131–144.
Ritter, C. E. T., Camassola, M., Zampieri, D., Silveira, M. M., & Dillon, A. J. P. (2013). Cellulase and xylanase production by Penicillium echinulatum in submerged media containing cellulose amended with sorbitol. Enzyme Research, 2013, 1–9. https://doi.org/10.1155/2013/240219
dos Santos, F. C., de Oliveira, M. A. S., Seixas, F. A. V., & Barbosa-Tessmann, I. P. (2020). A novel cellobiohydrolase I ( CBHI ) from Penicillium digitatum : Production, purification, and characterization. Applied Biochemistry and Biotechmology, 192, 257–282.
Prajapati, B. P., Kumar Suryawanshi, R., Agrawal, S., Ghosh, M., & Kango, N. (2018). Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresource Technology, 250, 733–740. https://doi.org/10.1016/j.biortech.2017.11.099
Liu, D., Zhang, R., Yang, X., Zhang, Z., Song, S., Miao, Y., & Shen, Q. (2012). Characterization of a thermostable b -glucosidase from Aspergillus fumigatus Z5, and its functional expression in Pichia pastoris X33. Microbial Cell Factories, 11(1), 25. https://doi.org/10.1186/1475-2859-11-25
Grigorevski-Lima, A. L., Da Vinha, F. N. M., Souza, D. T., Bispo, A. S. R., Bon, E. P. S., Coelho, R. R. R., & Nascimento, R. P. (2009). Aspergillus fumigatus thermophilic and acidophilic endoglucanases. Applied Biochemistry and Biotechnology, 155(1–3), 18–26. https://doi.org/10.1007/s12010-008-8482-y
Tao, Y. M., Zhu, X. Z., Huang, J. Z., Ma, S. J., Wu, X. B., Long, M. N., & Chen, Q. X. (2010). Purification and properties of endoglucanase from a sugar cane bagasse hydrolyzing strain, Aspergillus glaucus XC9. Journal of Agricultural and Food Chemistry, 58(10), 6126–6130. https://doi.org/10.1021/jf1003896
Imran, M., Anwar, Z., Zafar, M., Ali, A., & Arif, M. (2018). Production and Characterization of Commercial Cellulase Produced Through Aspergillus Niger Immis1 after Screening Fungal Species. Pakistan Journal of Botany, 50(27), 1563–1570. Retrieved from http://www.pakbs.org/pjbot/papers/1524266431.pdf. Accessed 22 Sep 2020.
Das, A., Jana, A., Paul, T., Halder, S. K., Ghosh, K., & Maity, C. (2013). Thermodynamics and kinetic properties of halostable endoglucanase from Aspergillus fumigatus ABK9. Journal of Basic Microbiology, 54, 1–10. https://doi.org/10.1002/jobm.201300350
Narra, M., Dixit, G., Divecha, J., Kumar, K., Madamwar, D., & Shah, A. R. (2014). Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. International Biodeterioration & Biodegradation, 88, 150–161. https://doi.org/10.1016/j.ibiod.2013.12.016
Moraes, A. D. O., Modesto, L. F., Isabel, N., Ramirez, B., & Pereira, N., Jr. (2016). Reuse of residual biomass of cellulose industry for second generation bioethanol production. Journal of Advances in Biotechnology, 6(1), 768–772.
Bayitse, R., Hou, X., Bjerre, A.-B., & Saalia, F. K. (2015). Optimisation of enzymatic hydrolysis of cassava peel to produce fermentable sugars. AMB Express, 5(1), 60. https://doi.org/10.1186/s13568-015-0146-z
Efeovbokhan, V. E., Egwari, L., Alagbe, E. E., Adeyemi, J. T., & Taiwo, O. S. (2019). Production of bioethanol from hybrid cassava pulp and peel using microbial and acid hydrolysis. BioResources, 14(2), 2596–2609. Retrieved from https://bioresources.cnr.ncsu.edu/resources/production-of-bioethanol-from-hybrid-cassava-pulp-and-peel-using-microbial-andacid-hydrolysis. Accessed 22 Sept 2020.
Jun, H., Kieselbach, T., & Jönsson, L. J. (2011). Enzyme production by filamentous fungi: Analysis of the secretome of Trichoderma reesei grown on unconventional carbon source. Microbial Cell Factories. https://doi.org/10.1186/1475-2859-10-68
Chen, C., Duan, C., Li, J., Liu, Y., & Ma, X. (2016). Cellulose (dissolving pulp) manufacturing processes. BioResources, 11(2), 5553–5564. Retrieved from https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_11_2_Review_Chen_Cellulose_Manufacturing_Processes. Accessed 25 Aug 2020.
Lopes, A. M., Filho, E. X. F., & Moreira, L. R. S. (2018). An update on enzymatic cocktails for lignocellulose breakdown. Journal of Applied Microbiology, 125(3), 632–645. https://doi.org/10.1111/jam.13923
Maeda, N. R., Isabel, V., Alves, V., Rocha, L., Aparecida, R., Mesquita, A., & São-carlense, A. T. (2011). Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicillium funiculosum and Trichoderma harzianum cellulases. Process Biochemistry, 46(5), 1196–1201.https://doi.org/10.1016/j.procbio.2011.01.022
Adsul, M., Sharma, B., Singhania, R. R., Saini, J. K., Sharma, A., Mathur, A., & Tuli, DK. (2014) Blending of cellulolytic enzyme preparations from different fungal sources for improved cellulose hydrolysis by increasing synergism. RSC Advances. 4(84), 44726–44732. https://doi.org/10.1039/C4RA08129C
Arias, J. M., Modesto, L. F. A., Polikarpov, I., & Pereira Jr., N. (2016). Design of an Enzyme Cocktail Consisting of Different Fungal Platforms for Efficient Hydrolysis of Sugarcane Bagasse: Optimization and Synergism Studies. Biotechnology Progress, 00, 1–8. https://doi.org/10.1002/btpr.2306
Agrawal, R., Semwal, S., Kumar, R., Mathur, A., & Gupta, R. P. (2018). Synergistic enzyme cocktail to enhance hydrolysis of steam exploded wheat straw at pilot scale 6 November 1 11. https://doi.org/10.3389/fenrg.2018.00122
Teter, S. A., Sutton, K. B., & Emme, B. (2014). Enzymatic processes and enzyme development in biorefining. In Advances in Biorefineries (pp. 199–233). Elsevier. https://doi.org/10.1533/9780857097385.1.199
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pimentel, P.S.SR., de Oliveira, J.B., Astolfi-Filho, S. et al. Enzymatic Hydrolysis of Lignocellulosic Biomass Using an Optimized Enzymatic Cocktail Prepared from Secretomes of Filamentous Fungi Isolated from Amazonian Biodiversity. Appl Biochem Biotechnol 193, 3915–3935 (2021). https://doi.org/10.1007/s12010-021-03642-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03642-5