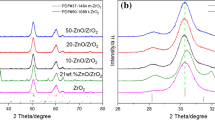
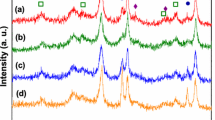
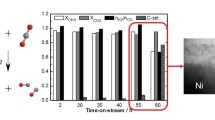
Abstract
ZnO/TiO2 catalysts, with different compositions, have been synthesized by wetness impregnation of TiO2 in Zn(NO3)2 water solution and by precipitation of ZnCO3 in the presence of TiO2, characterized by X-ray diffraction and surface area measurements and tested in the methanol steam reforming reaction. The methanol conversion, the hydrogen yield and the CO selectivity were compared for composition, for synthesis methods and for surface area. The activity resulted proportional to the surface area for both kind of preparations. Precipitated catalyst had higher surface area. Nevertheless, impregnation method gave better performances, in term of methanol conversion, hydrogen yield and CO selectivity. XRD gave evidence of a more crystalline structure for impregnated catalysts than for precipitated ones. This higher crystallinity allows for better electronic interactions between ZnO and TiO2, which is probably responsible for the better catalytic performance. Using GHVSCH3OH = 5 × 104 h−1 impregnated catalyst with χZn ≤ 0.22 reached methanol conversion completeness at T = 400 °C with CO selectivity = 2% and gave GHVSH2 = 14.5 × 104 h−1 at 400 °C and 4.5 × 104 h−1 at 350 °C. CO selectivity resulted invariant with respect to methanol conversion, giving information about possible reaction pathway.






Similar content being viewed by others
References
Song C (2002) Fuel processing for low temperature and high-temperature fuel cells: challenges, and opportunities for sustainable development in the 21st century. Catal Today. https://doi.org/10.1016/S0920-5861(02)00231-6
Ghenciu AF (2002) Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr Opin Solid State Mater Sci. https://doi.org/10.1016/S1359-0286(02)00108-0
Dutta S (2014) A review on production, storage of hydrogen and its utilization as an energy resource. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2013.07.037
Trimm DL, Önsan ZI (2001) Onboard fuel conversion for hydrogen-fuel-cell-driven vehicles. Catal Rev. https://doi.org/10.1081/CR-100104386
Günter MM, Ressler T, Jentoft RE, Bems B (2001) Redox behavior of copper oxide/zinc oxide catalysts in the steam reforming of methanol studied by in situ X-ray diffraction and absorption spectroscopy. J Catal. https://doi.org/10.1006/jcat.2001.3322
Matter PH, Braden DJ, Ozkan US (2004) Steam reforming of methanol to H2 over nonreduced Zr-containing CuO/ZnO catalysts. J Catal. https://doi.org/10.1016/j.jcat.2004.01.031
Wang LC, Liu YM, Chen M, Cao Y, He HY, Wu GS, Dai WL, Fan KN (2007) Production of hydrogen by steam reforming of methanol over Cu/ZnO catalysts prepared via a practical soft reactive grinding route based on dry oxalate-precursor synthesis. J Catal. https://doi.org/10.1016/j.cat.2006.12.006
Shishido T, Yamamoto Y, Morioka H, Takehira K (2007) Production of hydrogen from methanol over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation: steam reforming and oxidative steam reforming. J Mol Catal A. https://doi.org/10.1016/j.molcata.2006.12.018
Kniep BL, Girgsdies F, Ressler T (2005) Effect of precipitate aging on the microstructural characteristics of Cu/ZnO catalysts for methanol steam reforming. J Catal. https://doi.org/10.1016/j.jcat.2005.09.001
Shen GC, Fujita SI, Matsumoto S, Takezawa N (1997) Steam reforming of methanol on binary Cu/ZnO catalysts: effects of preparation condition upon precursors, surface structure and catalytic activity. J Mol Catal A. https://doi.org/10.1016/S1381-1169(97)00078-2
Mateos-Pedrero C, Silva H, Tanaka DAP, Liguori S, Iulianelli A, Basile A, Mendes A (2015) CuO/ZnO catalysts for methanol steam reforming: the role of the support polarity ratio and surface area. Appl Catal B. https://doi.org/10.1016/j.apcatb.2015.02.039
Purnama H, Ressler T, Jentoft RE, Soerijanto H, Schlögl R, Schomäcker R (2004) CO formation/selectivity for steam reforming of methanol with a commercial CuO/ZnO/Al2O3 catalyst. Appl Catal A. https://doi.org/10.1016/j.apcata.2003.09.13
Agrell J, Birgersson H, Boutonnet M (2002) Steam reforming of methanol over a Cu/ZnO/Al2O3 catalyst: a kinetic analysis and strategies for suppression of CO formation. J Power Sources. https://doi.org/10.1016/S0378-7753(01)01027-8
Breen JP, Ross JRH (1999) Methanol reforming for fuel-cell applications: development of zirconia-containing Cu-Zn-Al catalysts. Catal Today. https://doi.org/10.1016/S0920-5861(99)00038-3
Wan Y, Zhou Z, Cheng Z (2016) Hydrogen production from steam reforming of methanol over CuO/ZnO/Al2O3 catalysts: catalytic performance and kinetic modelling. Chin J Chem Eng 24:1186–1194. https://doi.org/10.1016/j.cjche.2016.02.006
Li J, Mei X, Zhang L, Yu Z, Liu Q, Wei T, Wu W, Dong D, Xu L, Hu X (2020) A comparative study of catalytic behaviors of Mn, Fe Co, Ni, Cu and Zn-based catalysts in steam reforming of methanol, acetic acid and acetone. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.03.269
Kim W, Mohaideen KK, Seo DJ, Yoon WL (2017) Methanol-steam reforming reaction over Cu-Al-based catalysts derived from layered double hydroxides. Int J Hydrogen Energy 42:2081–2087. https://doi.org/10.1016/j.ijhydene.2016.11.014
Matter PH, Ozkan US (2005) Effect of pretreatment conditions on Cu/Zn/Zr-based catalysts for the steam reforming of methanol to H2. J Catal. https://doi.org/10.1016/j.jcat.2005.07.007
Liu Y, Hayakawa T, Suzuki K, Hamakawa S, Tsunoda T, Ishii T, Kumagai M (2002) Highly active copper/ceria catalysts for steam reforming of methanol. Appl Catal A. https://doi.org/10.1016/S0926-860X(01)00733-5
Papavasiliou J, Avgouropoulos G, Ioannides T (2007) Effect of dopants on the performance of CuO–CeO2 catalysts in methanol steam reforming. Appl Catal B. https://doi.org/10.1016/j.apcatb.2006.07.007
Abrokwah RY, Deshmane VG, Kuila D (2016) Comparative performance of M-MCM-41 (M: Cu Co, Ni, Pd, Zn and Sn) catalysts for steam reforming of methanol. J Mol Catal A 425:10–20. https://doi.org/10.1016/j.molcata.2016.09.019
Deshmane VG, Abrokwah RY, Kuila D (2015) Synthesis of stable Cu-MCM-41 nanocatalysts for H2 production with high selectivity via steam reforming of methanol. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2015.06.084
Agrell J, Birgersson H, Boutonnet M, Melián-Cabrera I, Navarro RM, Fierro JLG (2003) Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3. J Catal. https://doi.org/10.1016/S0021-9517(03)00221-5
Zhang L, Pan L, Ni C, Sun T, Zhao S, Wang S, Wang A, Hu Y (2013) CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming. Int J Hydrogen Energy. https://doi.org/10.1016/j.ihydene.2013.01.053
Patel S, Pant KK (2006) Activity and stability enhancement of copper–alumina catalysts using cerium and zinc promoters for the selective production of hydrogen via steam reforming of methanol. J Power Sources. https://doi.org/10.1016/j.jpowsour.2006.04.008
Mrad M, Hammoud D, Gennequin C, Aboukaïs A, Abi-Aad E (2014) A comparative study on the effect of Zn addition to Cu/Ce and Cu/Ce–Al catalysts in the steam reforming of methanol. Appl Catal A. https://doi.org/10.1016/j.apcata.2013.11.025
Mohtashami Y, Taghizadeh M (2019) Performance of the ZrO2 promoted Cu-ZnO catalyst supported on acetic acid-treated MCM-41 in methanol steam reforming. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.01.029
Iwasa N, Mayanagi T, Ogawa N, Sakata K, Takezawa N (1998) New catalytic functions of Pd-Zn, Pd-Ga, Pd-In, Pt-Zn, Pt-Ga and Pt-In alloys in the conversions of methanol. Catal Lett. https://doi.org/10.1023/A:1019056728333
Ranganathan ES, Bej SK, Thompson LT (2005) Methanol steam reforming over Pd/ZnO and Pd/CeO2 catalysts. Appl Catal A. https://doi.org/10.1016/j.apcata.2005.04.022
Chin Y-H, Dagle R, Hu J, Dohnalkova AC, Wang Y (2002) Steam reforming of methanol over highly active Pd/ZnO catalyst. Catal Today. https://doi.org/10.1016/S0920-5861(02)00234-1
Iwasa N, Takezawa N (2003) New supported Pd and Pt alloy catalysts for steam reforming and dehydrogenation of methanol. Top Catal. https://doi.org/10.1023/A:1023571819211
Takezawa N, Iwasa N (1997) Steam reforming and dehydrogenation of methanol: difference in the catalytic functions of copper and group VIII metals. Catal Today. https://doi.org/10.1016/S0920-5861(96)00195-2
Dagle RA, Platon A, Palo DR, Datye AK, Vohs JM, Wang Y (2008) PdZnAl catalysts for the reactions of water-gas-shift, methanol steam reforming, and reverse-water-gas-shift. Appl Catal A. https://doi.org/10.1016/j.apcata.2008.03.005
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev. https://doi.org/10.1021/cr900356p
Fujishima A, Zhang X, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep. https://doi.org/10.1016/j.surfrep.2008.10.001
Hernandez-Alonso MD, Fresno F, Suarez S, Coronado JM (2009) Development of alternative photocatalysts to TiO2: challenges and opportunities. Energy Environ Sci. https://doi.org/10.1039/b907933e
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev. https://doi.org/10.1021/cr1001645
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res. https://doi.org/10.1016/j.watres.2015.09.045
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C. https://doi.org/10.1016/j.jphotochemrev.2007.12.003
Yoo SJ, Lee H-S, Veriansyah B, Kim J, Kim J-D, Lee Y-W (2010) Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour Technol. https://doi.org/10.1016/j.biortech.2010.06.073
Madhuvilakku R, Piraman S (2013) Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour Technol. https://doi.org/10.1016/j.biortech.2013.09.087
Pinzari F, Patrono P, Costantino U (2006) Methanol reforming reactions over Zn/TiO2 catalysts. Catal Commun. https://doi.org/10.1016/j.catcom.2006.02.015
Deshmane VG, Owen SL, Abrokwah RY, Kuila D (2015) Mesoporous nanocrystalline TiO2 supported metal (Cu Co, Ni, Pd, Zn, and Sn) catalysts: effect of metal-support interactions on steam reforming of methanol. J Mol Catal A. https://doi.org/10.1016/j.molcata.2015.07.023
Gribovskii AG, Makarshin LL, Andreev DV, Korotaev SV, Dutov PM, Khantakov RM, Reshetnikov SI, Parmon VN (2009) Efficiency of Zn/TiO2 catalyst operation in a microchannel reactor in methanol steam reforming. Kinet Catal. https://doi.org/10.1134/S0023158409010029
Gribovskii AG, Makarshin LL, Andreev DV, Korotaev SV, Zaikovskii VI, Parmon VN (2009) Deactivation of a Zn/TiO2 catalyst in the course of methanol steam reforming in a microchannel reactor. Kinet Catal. https://doi.org/10.1134/S0023158409030161
César DV, Robertson RF, Resende NS (2008) Characterization of ZnO and TiO2 catalysts to hydrogen production using thermoprogrammed desorption of methanol. Catal Today. https://doi.org/10.1016/j.cattod.2007.12.089
Chang YS, Chang YH, Chen IG, Chen GJ, Chai YL (2002) Synthesis and characterization of zinc titanate nano-crystal powders by sol–gel technique. J Cryst Growth. https://doi.org/10.1016/S0022-0248(02)01490-2
Yu YH, Xia M (2012) Preparation and characterization of ZnTiO3 powders by sol–gel process. Mater Lett. https://doi.org/10.1016/j.matlet.2012.02.113
Reyes-Coronado D, Rodrıguez-Gattorno G, Espinosa-Pesqueira ME, Cab C, de Coss R, Oskam G (2008) Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology. https://doi.org/10.1088/0957-4484/19/14/145605
Yu JC, Yu J, Ho W, Jiang Z, Zhang L (2002) Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem Mater. https://doi.org/10.1021/cm020027c
Zheng JH, Jiang Q, Lian JS (2011) Synthesis and optical properties of flower-like ZnO nanorods by thermal evaporation method. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2011.01.025
Ginés MJL, Marchi AJ, Apesteguía CR (1997) Kinetic study of the reverse water-gas shift reaction over CuO/ZnO/Al2O3 catalysts. Appl Catal A. https://doi.org/10.1016/S0926-860X(96)00369-9
Acknowledgements
We wish to thank Dr. P. Caffarelli for Surface Area and AA measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinzari, F. The effect of the preparation on the catalytic activity of ZnO/TiO2 in the methanol steam reforming reaction. Reac Kinet Mech Cat 134, 23–35 (2021). https://doi.org/10.1007/s11144-021-02044-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02044-2