Abstract
The samples of humic preparations (HPs) containing macro and trace elements for plant nutrition were obtained based on humic acids isolated from brown coal of the Tisul deposit in the Kansk-Achinsk Coal Basin. The biological activity of the humic preparations was tested under laboratory and field conditions with the use of Iren and Novosibirskaya 89 varietal wheat seeds. A comparative assessment of the effects of the concentrations of various elements in the HPs on the growth parameters and productivity of cereals was carried out. The edaphic properties of soil substrates should be taken into account for a more effective use of humic preparations.
Similar content being viewed by others
INTRODUCTION
Currently, the yield and quality of agricultural products cannot be increased without the use of advanced technologies, including the use of biologically active substances. Recently, interest has increased in the use of humic acids (HAs) and preparations based on them as plant growth stimulants in agriculture. With the use of humates in agriculture, the yields of grain, fodder, and vegetable crops increased by an average of 10–30%; the germination of seeds was increased, the metabolism of plants was improved, and the root formation was enhanced. The ability of humic substances (HSs) to stimulate plant growth has been confirmed by many studies carried out using a variety of plant species cultivated under various conditions [1]. This beneficial effect of HSs on the development of plants is reflected in both the growth of roots and seedlings and in the yield. However, the mechanisms responsible for this action of humic substances are only partially known and poorly systematized. There is a point of view that the mechanisms of influence of HSs on root growth and seedling growth are different [2]. The structure of HSs, the presence of aromatic and aliphatic domains, which include O-, N-, and S-containing functional groups, mobility, aromaticity, and hydrophobicity can affect the activity of enzymes, the stimulation of root growth and increase of the biomass. Labile HS fragments can reach root surfaces and interact with cell membranes, regulate cell metabolism, and increase the activity of glycolysis enzymes [2, 3]. HAs affect the enzymatic activity of plant cells and enhance photochemical processes, electron transport, and phosphorylation [1, 4]. At the same time, the permeability of the cell membrane increases to facilitate the penetration of nutrients and trace elements into the cell and speeds up the respiration of plants. The entry of HAs in a dissolved state into the plant cell is accompanied by an increase in redox reactions according to the Bach–Paladin–Sent-Györgyi theory: hydroquinone bianion ↔ radical anion ↔ benzoquinone. The presence of free radicals increases the energy reserve and the activity of the enzymatic system in the plant cell to increase its overall metabolism. Humic acids penetrate into both individual plant organs and cell organelles: chloroplasts, mitochondria, and nuclei. HAs have a positive effect on the acceleration of RNA synthesis; they accelerate protein synthesis in general. After the application of HSs, an increased concentration of amino acids (glutamate, aspartate, serine, glycine, and methionine) was found in plant leaves [5]. A number of humic substances obtained from various sources have been investigated. Biological activity was assessed by changes in root structure and activation of the proton pump in tomato and corn as examples. It was assumed that the hydrophobic domain of HSs contains biologically active molecules, like auxins, which promote root growth [6]. However, Conselvan et al. [4] concluded that the domain of HSs, which consists of aromatic hydrocarbons and amide functional groups, acts like gibburellic acid, that is, as a growth hormone, and the presence of carboxyl groups is an indicator of the bioavailability of auxin-like molecules.
Thus, at present, there are several points of view on the mechanism of the stimulating effect of humic substances on plants. It can be assumed that the stimulating effect of HSs on plants is a result of many simultaneous biological and chemical processes that depend primarily on the properties of HSs and the chemical and biological properties of soil and the plants themselves.
The biological activity of humic preparations can be enhanced by introducing macro and trace elements into humates for plant nutrition [7].
The aim of this work was to study the biological activity of complex humic preparations based on native (HumNa) humic acids and humic acids modified with hydrogen peroxide and also containing macro and trace elements.
EXPERIMENTAL
Humic acids from humus brown coals of the Tisul deposit in the Kansk-Achinsk Basin were selected for this study. Humates were prepared by alkaline extraction with a 1% solution of NaOH according to a standard procedure [8]. Modification with hydrogen peroxide was carried out as follows: 50 mL of distilled water was added to a glass beaker with 150 mL of the obtained native sodium humate (3.5%). With constant stirring on a magnetic stirrer, 10 mL of Н2О2 (32%) was slowly added dropwise from a burette. After the last drop of hydrogen peroxide was added, stirring was continued for another 10 min.
Tests were carried out to determine the biological activity of humic acids in the form of initial sodium humates of different concentrations and humates with the addition of various macro and trace elements. Peive [7] proposed the forms and doses of fertilizers containing various macro and trace elements to increase productivity taking into account the type of soil, the type of plant culture, and the concentrations of elements in plants. For the experiments, aqueous solutions of sodium humates modified with hydrogen peroxide [9] (HP 1) and humates containing macro and trace elements (HP 2) and (HP 3), cobalt (HP 4), and manganese (HP 5) were prepared in accordance with Table 1. The following chemicals were used for the preparations: carbamide (NH2)2CO, potassium sulfate K2SO4, calcium nitrate Ca(NO3)2, sodium metaborate Na2BO4 ⋅ 4H2O, ammonium molybdate (NH4)6Mo7O24 ⋅ 4H2O, manganese sulfate MnSO4 ⋅ 5H2O, copper nitrate Cu(NO3)2·3H2O, cobalt nitrate Co(NO3)2 ⋅ 6H2O, and zinc nitrate Zn(NO3)2 ⋅ 3H2O. The weighed portions of the substances added to humate solutions were calculated based on the total percentage of active components (Table 1).
The experiments were carried out using published procedures [10, 11] in accordance with GOST [State Standard] 12038-84 and GOST R 54221-2010 [12, 13]. The tests were carried out under laboratory and field conditions.
The biological activity was evaluated by phytotesting with cereals because they are used for fixing (sodding) the soil surface in the fight against desertification. For this purpose, Novosibirskaya 89 and Iren varietal spring wheat seeds were taken. The choice of sowing wheat was justified by the fact that wheat is a widespread annual crop, the study of which provides quantitative and qualitative data based on the results of cultivation.
The biological activity was estimated based on the phytoactivity index (PI) taking into account the seed germination energy (GE), the root length (RL) as an indicator reflecting the plant response to the concentration of biogenic elements, and the sprout height (SH) as an indicator that makes it possible to assess the phytohormonal effect of a preparation. The experiments were repeated three times. The value of PI is a generalized index, which reflects deviations of the test function from a control value and is calculated as
where GE, RL, and SH are average values, % on a control basis [10]. Simultaneously, wheat seeds were soaked in distilled water under the same conditions as a control experiment. In some experiments, the number of roots (NR) was additionally measured.
All batches of seeds were preliminarily tested for germination. Batches with a seed germination rate of at least 90% were selected for the experiments. Statistical processing of the experimental data was carried out by the determination of the Spearman’s rank correlation coefficient using the Microsoft Office Excel, PAST V2.17 software package.
RESULTS AND DISCUSSION
Laboratory studies. Since a concentrated solution of humic acids can negatively affect the growth and development of plants, its concentration was diluted to 0.02%. The pH of the solutions was 6.5–8.2, and it was within an acceptable range for the culture used. The seeds (20 pieces) were placed in vessels (Petri dishes) filled with the tested HP solutions or water (in the case of a control) before the experiments. Disks of filter paper were preliminarily placed on the bottom of the vessels. The solutions of the preparations were introduced into the vessels so that the liquid completely overlapped the seeds but by no more than 3 mm. After that, the vessels were transferred to a thermostat (t = 26°C), where they were kept in the dark for 72 h.
The results of laboratory tests showed that all of the evaluated preparations exerted a positive effect on the germination energy of wheat seeds (Table 2). In this case, humic preparations with the addition of cobalt (HP 4) and manganese (HP 5) exhibited maximum biological activity in relation to this characteristic. These preparations also showed a maximum positive effect on the root length. Negative effects on the root length and the sprout height were obtained with the use of HP 3 and HP 2 preparations, respectively.
The calculation and statistical processing of the integral phytoactivity indexes (Fig. 1) showed the following order of efficiency: HP 4 > HP 5 > HP 1 > HP 3 > HP 2. Preparations in the solutions of which no biogenic elements were present exhibited maximum biological activity. In a version with the addition of H2O2 (HP 1), the values were slightly higher than the control values, probably, due to the oxidation of aliphatic fragments of humic acids, which are responsible for their biological activity, by peroxide. Minimum values of the phytoactivity index were characteristic of HP 2 and HP 3 preparations enriched in both macro and trace elements. The availability of biogenic elements, on the one hand, does not stimulate the development of plant root systems and, on the other hand, facilitates the neutralization of the action of humic acids in preparations containing calcium (HP 2) [14].
To establish the concentration dependence of PI, 0.0005, 0.005, and 0.01% solutions (in terms of sodium humate) of humic preparations were prepared from the initial solutions. In this case, the concentrations of all macro and trace elements changed in proportion to dilution. In this experiment, the seeds of Iren wheat were soaked in grower trays, 50 pieces in each tray. Table 3 summarizes the phytotesting data.
Comparing the data in Tables 2 and 3, we can conclude that the use of the HP 2 preparation with concentrations of 0.02 and 0.01% exerted almost the same effects close to that in the control. Moreover, in both cases, the preparation had a depressing effect on the height of seedlings and also on the length of the root at a concentration of 0.01%. The HP 2 preparation at a minimum concentration (0.0005%) exerted a maximum positive effect on seed germination. The root length and sprout height increased by 17 and 11%, respectively, compared to the control. The values of PI exceed the control ones by 11.2%. It is likely that the degree of neutralization of the action of humic acids decreased with a decrease in the CaO content.
The maximum positive effects of the HP 4 and HP 5 preparations were manifested at a sodium humate concentration of 0.005% (PI = 1.22 and 1.24, respectively). The sprout heights obtained with the use of these preparations exceeded the control by 52 and 53%, respectively.
The solutions of sodium humate (0.005%) containing cobalt and manganese cations, the concentrations of which varied from 0.001 to 0.1%, were prepared for further experiments because it was experimentally found previously that humic preparations containing cobalt and manganese exhibited maximal biological activity. Table 4 summarizes the experimental results, which indicate the positive effect of sodium humate on the growth characteristics of wheat seeds (PI was higher by 39% than the control value). The preparations containing manganese and cobalt at a concentration of 0.001% exhibited maximal biological activity (PI of 1.5 and 1.49, respectively). These preparations exerted a positive effect on all test functions, which exceed control values by more than 50%. An increase in the concentration of manganese and cobalt led to a decrease in the values of PI. The lowest values were observed in experiments performed using preparations with cobalt contents of 0.05 and 0.1%. In this case, the root length and the sprout height were lower than those in the control experiments with water. It should be noted that preparations containing cobalt affected an increase in the number of seedling roots, while the formation of lateral roots was observed with the use of manganese. The use of preparations based on sodium humate and containing the cations of cobalt and manganese promoted the development of the root system of plants, which is an important factor for strengthening the structure of soil.
Field experiments. The field tests of humic preparations were carried out in two variants: with pre-sowing seed treatment and with watering of seedlings (0.02% of sodium humate). The studies were carried out in the areas of anthropogenic landscapes and dump of the Zarechny coal mine of AO SUEK-Kuzbass. The selection of sites for field experiments was based on those properties that correspond to arid extracontinental regions of Mongolia. Therefore, substrates represented by anthropogenic eluvium of dense coal-bearing rocks similar to stony soils of Mongolia and loess-like carbonate loams—protruding sedimentary rocks as a model of loamy and clayey soils in arid regions—were selected when laying the experimental sites. The use of these substrates makes it possible to more reliably evaluate the effect of humic preparations due to an insignificant concentration of organic carbon in them (to 3%).
In variants with the treatment of seeds, they were soaked in the solutions of preparations for a day and then sown. In variants with seedling treatment, watering was carried out two weeks after sowing. The experiment was repeated five times on plots with a surface area of 2 m2. Distilled water was used instead of humate solutions in control experiments in the same volumes as those in the experiments with preparations. In all variants, the resulting effect of humic preparations was assessed by their effect on the yield of cereal plants, which was determined at the end of the growing season based on the amount of dry phytomass. Novosibirskaya 89 spring wheat variety was chosen as a crop.
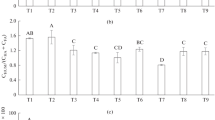
The results of field tests showed that the effect of humates on the value of the phytomass of cereal plants was manifested unequally and depended on the edaphic properties (the physical and chemical properties of substrates in the plots). Because of this, the yield of dry phytomass in the plots of the field experiment was low and ranged from 2 to 8 hkg/ha. At the same time, the effect of the preparations in a number of variants was positive in relation to the control variants, in which distilled water was used instead of humates (Fig. 2).
Humic preparations exerted the most pronounced effect on the substrates of stony rocks. It should be noted that, under conditions of moisture deficiency inherent in these rocks, HP 4 and HP 5 containing cobalt and manganese, respectively, were among the most effective humates. The effect of HP 2 manifested itself at almost the same level of efficiency; this was most likely due to the presence of macro elements balanced in composition in solutions (including nitrogen, phosphorus, potassium, and calcium) in addition to the influence of Co and Mn. In the case of HP 3, which was also enriched in Co, Mn, N, and K, but without P and Ca, the effect of the preparation was minimal and lower than the effect of HP 1, that is, humate modified with hydrogen peroxide.
In general, the effects of humic preparations on the phytomass of wheat grown on stony rocks were similar both for the versions with seed soaking and for plots where the preparations were applied with irrigation.
On the experimental sites composed of loamy rocks, the effects of humates were manifested unequally. Under conditions of greater moisture, negative effects of humic preparations were detected with the use of HP 4 and HP 5 (Fig. 2). Also negative but less pronounced effects of the use of HP 1 and HP 2 were noted in the versions with irrigation. At the same time, HP 3 exerted a maximum positive effect on the phytomass of wheat. Obviously, the observed effect was associated with the presence of sulfur in this modification and the highest nitrogen content (Table 1) because the absence of phosphorus and potassium from the preparation was compensated by their presence in plot substrates. In the versions when seeds were soaked, the effect of HP 3 was lower than that of the use of HP 1 and HP 2; nevertheless, the yields in this case exceeded the control values by an average of 13%.
CONCLUSIONS
Thus, the experimental studies demonstrated that humates containing macro and trace elements had different effects on the growth characteristics of cereal plants. Laboratory tests allowed us to establish that the biological activity depends on the concentration of the preparations used and on the concentrations of macro and trace elements in them. The preparations (0.005% in terms of humate) containing cobalt and manganese (0.001%) exhibited maximum biological activity. Preparations in the solution of which biogenic elements, especially calcium, which neutralizes the effect of humic acids, were present exhibited minimum biological activity. Field tests have shown that the modifications of humic preparations used in the fight against desertification should be chosen taking into account the edaphic and climatic conditions of the territory of their application. At the same time, the greatest effect of the use of solutions of humic preparations was manifested on stony soils experiencing an acute deficit of moisture, where almost all of the test preparations had a positive effect.
REFERENCES
Olaetxea, M., De Hita, D., Garcia, C.A., Fuentes, M., Baigorri, R, Mora, V., Garnica, M., Urrutia, O., Erro, J., Zamarreño, A.M., Berbara, R., and García-Mina, J.M., Appl. Soil Ecol., 2018, vol. 123, p. 521.
Garcia, A.C., Van Tol, De., Castro, T.A., Santos, L.A., Carlos, O., Tavares, H., Castro, R.N., Berbara, R., Luiz, L., and Garcia-Mina, J.M., J. Environ. Quality, 2019, vol. 48, no. 6, p. 1622.
Zandonadi, D.B., Matos, C.R., Castro, R.N., Spaccini, R., Olivares, F.L., and Canellas, L.P., Chem. Biol. Technol. Agricult., 2019, vol. 6, no. 1, p. 23.
Conselvan, G.D., Pizzeghello, D., Francioso, O., Foggia, M.D., Nardi, S., and Carletti, P., Plant Soil, 2017, vol. 420, p. 119.
Calvo, P., Nelson, L., and Kloepper, J.W., Plant Soil, 2014, vol. 383, p. 3.
Dobbs, L.B., Canallas, L.P., Olivares, F.L., Aguiar, N., Peres, L., Azevedo, M., Spaccini, R., Piccolo, A., and Facanha, A.R., Agricult. Food Chem., 2010, vol. 58, p. 3681.
Peive, Ya.V., Rukovodstvo po primeneniyu mikroudobrenii (Microfertilizer Application Guide), Moscow: Sel’khozizdat, 1963.
Taits, E.M. and Andreeva, I.A., Metody analiza i ispytaniya uglei (Methods for the Analysis and Testing of Coals), Moscow: Nedra, 1983.
Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S., Shpakodraev, K.M., and Ismagilov, Z.R., Solid Fuel Chem., 2020, vol. 54, no. 4, p. 191. https://doi.org/10.3103/S0361521920040096
Voronina, L.P, Yakimenko, O.S., and Terekhova, V.A., Agrokhimiya, 2012, no. 6, p. 50.
Vavilov, P.P., Gritsenko, V.V., and Kuznetsov, V.S., Praktikum po rastenievodstvu (Crop Manual), Moscow: Kolos, 1983.
GOST (State Standard) 12038-84: Crop Seeds: Methods for Determining Germination, Moscow: Izd. Standartov, 1984, p. 30.
GOST (State Standard) R 54221-2010: Humic Preparations from Brown and Oxidized Coals: Test Methods, Moscow: Standartinform, 2012, p. 10.
Ponamoreva, V.V. and Plotnikova, T.A., Gumus i pochvoobrazovanie (metody i rezul’taty izucheniya) (Humus and Soil Formation: Methods and Results of the Study), Leningrad: Nauka, 1980.
Funding
This work was supported by the Russian Foundation for Basic Research (grant no. 18-55-91033) and performed within the framework of a state contract at the Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences (project no. 121031500124-2).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zherebtsov, S.I., Malyshenko, N.V., Votolin, K.S. et al. Dependence of the Biological Activity of Brown Coal Humic Acids on the Concentrations of Macro and Trace Elements. Solid Fuel Chem. 55, 223–228 (2021). https://doi.org/10.3103/S036152192104011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S036152192104011X