Abstract
Objective
Recent data have shown that regular exercise may ameliorate motor symptoms in Parkinson’s disease (PD). This study aims to investigate how intended exercise impacts motor and non-movement symptoms of PD.
Methods
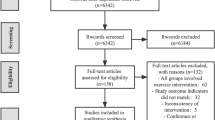
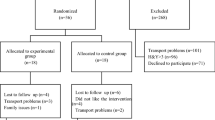
Eighty-eight patients were randomly assigned to an early exercise group (E-EG), late exercise group (L-EG), or a control group (CG) using a randomized delayed-start design. The E-EG carried out a rigorous, formal exercise program for 1 h, twice per week, for 18 months (May 2018–November 2019). The L-EG took part in the exercise program in the final 6–12 months of the study. We assessed outcomes using the Unified Parkinson’s Disease Rating Scale (UPDRS), PDQ-39 Questionnaire, Line A test, Line B test, Nine-hole column test, 30 s squat and stand-up test (30 s SST), 10-m walk test (10mW), Balance Evaluation Systems Mini Test (MiniBESTest), FAB, and Time Up and Go Test (TUG).
Results
The patients with PD in the E-EG had lower performance in the UPDRS and Line B test compared to those in the L-EG at post-exercise (p < 0.05). Moreover, the patients with PD in the E-EG had much lower performance in the PDQ-39 and 9-Hole Peg test compared to those in the L-EG at post-exercise (p < 0.01).
Conclusion
Implementation of an exercise regimen improved the movement abilities and quality of life in PD patients, especially in the E-EG. This data supports the idea that intended exercise should be implemented as part of the treatment strategy for PD patients as early as possible.



Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- E-EG:
-
Early-exercise group
- L-EG:
-
Late-exercise group
- CG:
-
Control group
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
- PDQ-39:
-
PDQ-39 Questionnaire
- 30 s SST:
-
30-S squat and stand-up test
- 10mW:
-
10-M walk test
- MiniBESTest:
-
Balance Evaluation Systems Mini Test
- TUG:
-
Time Up and Go Test
- FAB:
-
Fullerton advanced balance scale
- ADLs:
-
Activities of daily living
- LSVT-LOUD:
-
Lee Silverman Voice Treatment-LOUD
- H-Y scale:
-
Hoehn and Yahr scale
- LEDD:
-
Levodopa-equivalent daily dose
- fMRI:
-
Functional magnetic resonance imaging
References
King LA, Peterson DS, Mancini M, Carlson-Kuhta P, Fling BW, Smulders K et al (2015) Do cognitive measures and brain circuitry predict outcomes of exercise in Parkinson disease: a randomized clinical trial. BMC Neurol 15:218–227
Kulisevsky J, Oliveira L, Fox SH (2018) Update in therapeutic strategies for Parkinson’s disease. Curr Opin Neurol 31:439–447
Pellkofer HL, Krumbholz M, Berthele A, Hemmer B, Gerdes LA, Havla J et al (2011) Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 76:1310–1315
Ds B (1956) Establishing physical objectives in patients with Parkinson’s disease gymnasium activities. Phys Ther Rev 36:6
Ramig LSS, Countryman S, Pawlas A, O’Brien C, Hoehn M, Thompson L (2001) Intensive voice treatment (LSVT) for individuals with Parkinson disease: a two-year follow-up. J Neurol Neurosurg Psychiatry 71:6
McDonnell MN, Rischbieth B, Schammer TT, Seaforth C, Shaw AJ, Phillips AC (2018) Lee Silverman Voice Treatment (LSVT)-BIG to improve motor function in people with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil 32:607–618
Park A, Zid D, Russell J, Malone A, Rendon A, Wehr A et al (2014) Effects of a formal exercise program on Parkinson’s disease: a pilot study using a delayed start design. Parkinsonism Relat Disord 20:106–111
Krumpolec P, Vallova S, Slobodova L, Tirpakova V, Vajda M, Schon M et al (2017) Aerobic-strength exercise improves metabolism and clinical state in Parkinson’s disease patients. Front Neurol 8:12
Silveira CRA, Roy EA, Intzandt BN, Almeida QJ (2018) Aerobic exercise is more effective than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain Cogn 122:1–8
Ahlskog JE (2018) Aerobic exercise: evidence for a direct brain effect to slow Parkinson disease progression. Mayo Clin Proc 93:360–372
Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL (2008) The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 23:631–640
Mak MK, Wong-Yu IS, Shen X, Chung CL (2017) Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13:689–703
Tanaka K, Quadros AC Jr, Santos RF, Stella F, Gobbi LT, Gobbi S (2009) Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn 69:435–441
Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG (2011) Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand 123:13–19
Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW (2013) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. The Lancet Neurology 12:716–726
Diez-Cirarda M, Ojeda N, Pena J, Cabrera-Zubizarreta A, Lucas-Jimenez O, Gomez-Esteban JC et al (2018) Long-term effects of cognitive rehabilitation on brain, functional outcome and cognition in Parkinson’s disease. Eur J Neurol 25:5–12
Szeto Jennifer YY, Lewis Simon JG (2016) Current treatment options for Alzheimer’s disease and Parkinson’s disease dementia. Curr Neuropharmacol 14:326–339
Moritz CT (2018) Now is the critical time for engineered neuroplasticity. Neurotherapeutics 15:628–634
Khan F, Amatya B, Galea MP, Gonzenbach R, Kesselring J (2016) Neurorehabilitation: applied neuroplasticity. J Neurol 264:603–615
Zigmond MJ, Smeyne RJ (2014) Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord 20:S123–S127
Monteiro-Junior RS, Cevada T, Oliveira BRR, Lattari E, Portugal EMM, Carvalho A et al (2015) We need to move more: neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses 85:537–541
Acknowledgements
This study was carried out with the adequate understanding and consent of the patients. We would like to thank all participants and their families. We thank Prof. Yanhong Tai (Department of Neuropathology, The 5th Medical Center of Chinese PLA General Hospital, Beijing, China) for statistical assistance of the article. We also thank Prof. Yanchen Xie who provided professional writing services and partial materials.
Funding
This research received the grant from healthcare funding agency in the military scientific research institute (Military health-care project, No. 18BJZ34). The funding supported the design of the study, material collection, analysis, and interpretation of data and manuscript writing.
Author information
Authors and Affiliations
Contributions
Data analysis: Yang Yang, Jiarui Yao, Dan Liu, and Na Wang. Investigation: Yang Yang, Jiarui Yao, Na Wang, Tianyu Jiang, Yuliang Wang, and Dandan Liu. Methodology: Lifeng Chen and Weiping Wu. Formal design: Weiping Wu, Lifeng Chen, Tianyu Jiang, and Zhenfu Wang. Writing—review and editing: Yang Yang and Jiarui Yao. The author Pro. Tianyu Jiang who works in the Department of Rehabilitation Medicine, has taken part in all the exercise training, and endorsed by funding in the project (No.18BJZ34). The author Pro. Lifeng Chen who works in the Department of Neurosurgery, has done a lot work including curation of the data, polish of the revision of our manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee in General Hospital of Chinese PLA (IRB No. S2020-042–02).
Consent for publication.
The authors have consent for publication and have no conflict of interest to report.
Competing interests
The authors declare no competing interests.
Instructions for authors.
The authors have no conflict of interest to report. Our data availability statement has no conflict.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors Yang Yang and Lifeng Chen have contributed equally to this work
Rights and permissions
About this article
Cite this article
Yang, Y., Chen, L., Yao, J. et al. Early implementation of intended exercise improves quality of life in Parkinson’s disease patients. Neurol Sci 43, 1761–1767 (2022). https://doi.org/10.1007/s10072-021-05530-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05530-6