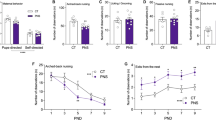
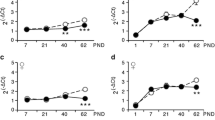
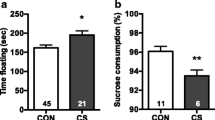
Abstract
Prenatal stress (PS) is a major risk factor for the development of emotional disorders in adulthood that may be mediated by an altered hypothalamic–pituitary–adrenal axis response to stress. Although the early onset of stress-related disorders is recognized as a major public health problem, to date, there are relatively few studies that have examined the incidence of early-life stressors in younger individuals. In this study, we assessed PS impact on the stress-coping response of juvenile offspring in behavioral tests and in the induced molecular changes in the hippocampus. Furthermore, we assessed if pregnancy stress could be driving changes in patterns of maternal behavior during early lactation. We found that PS modified stress-coping abilities of both sex offspring. In the hippocampus, PS increased the expression of bdnf-IV and crfr1 and induced sex difference changes on glucocorticoids and BDNF mRNA receptor levels. PS changed the hippocampal epigenetic landscape mainly in male offspring. Stress during pregnancy enhanced pup-directed behavior of stressed dams. Our study indicates that exposure to PS, in addition to enhanced maternal behavior, induces dynamic neurobehavioral variations at juvenile ages of the offspring that should be considered adaptive or maladaptive, depending on the characteristics of the confronting environment. Our present results highlight the importance to further explore risk factors that appear early in life that will be important to allow timely prevention strategies to later vulnerability to stress-related disorders.







Similar content being viewed by others
Data Availability
The data support the findings of this research are available from the corresponding author (mpallares@fmed.uba.ar) upon reasonable request. The authors will take the responsibility for maintaining availability.
Abbreviations
- 5-hmC:
-
5-Hydroxymethylcytosine
- 5-mC:
-
5-Methylcytosine
- ANOVA:
-
Analysis of variance
- BDNF:
-
Brain-derived neurotrophic factor
- C:
-
Control group
- CRF:
-
Corticotrophin-releasing factor
- CRFR1:
-
Corticotrophin-releasing factor receptor 1
- DNMT:
-
DNA methyl transferase
- EPM:
-
Elevated plus maze behavioral test
- FI:
-
Fragmentation index for maternal behavior assessment
- FST:
-
Forced swimming behavioral test
- GR:
-
Glucocorticoid receptor
- HPA:
-
Hypothalamic-pituitary-adrenal axis
- LDB:
-
Light-dark box behavioral test
- MR:
-
Mineralocorticoid receptor
- p75-NTR:
-
P75 neurotrophin receptor
- PND:
-
Postnatal day
- PS:
-
Prenatal stress group
- qPCR:
-
Polymerase chain reaction
- RA:
-
Risk assessment
- SHRP:
-
Stress hypo-responsive period
- SEM:
-
Standard error of the mean
- TET:
-
Ten-eleven translocation proteins
- TrkB:
-
Neurotrophic receptor tyrosine kinase 2
References
van Bodegom M, Homberg JR, Henckens MJ (2017) Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. www.frontiersin.org 11:87. https://doi.org/10.3389/fncel.2017.00087
McEwen BS (2016) In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann N Y Acad Sci 1373:56–64. https://doi.org/10.1111/nyas.13020
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. https://doi.org/10.1038/nrn2639
Koenig JI, Walker CD, Romeo RD, Lupien SJ (2011) Effects of stress across the lifespan. Stress 14:475–480. https://doi.org/10.3109/10253890.2011.604879
Roth TL, David Sweatt J (2011) Epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiatry Allied Discip 52:398–408. https://doi.org/10.1111/j.1469-7610.2010.02282.x
Chen Y, Baram TZ (2016) Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41:197–206. https://doi.org/10.1038/npp.2015.181
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18. https://doi.org/10.1016/S0149-7634(03)00005-8
Cottrell EC, Seckl JR (2009) Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3:1–9. https://doi.org/10.3389/neuro.08.019.2009
Beydoun H, Saftlas AF (2008) Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol 22:438–466. https://doi.org/10.1111/j.1365-3016.2008.00951.x
Boersma GJ, Tamashiro KL (2015) Individual differences in the effects of prenatal stress exposure in rodents. Neurobiol Stress 1:100–108. https://doi.org/10.1016/j.ynstr.2014.10.006
Buschdorf JP, Meaney MJ (2016) Epigenetics/programming in the HPA axis. Compr Physiol 6:87–110. https://doi.org/10.1002/cphy.c140027
Darnaudéry M, Dutriez I, Viltart O et al (2004) Stress during gestation induces lasting effects on emotional reactivity of the dam rat. Behav Brain Res 153:211–216. https://doi.org/10.1016/j.bbr.2003.12.001
Boulle F, Pawluski JL, Homberg JR et al (2016) Developmental fluoxetine exposure increases behavioral despair and alters epigenetic regulation of the hippocampal BDNF gene in adult female offspring. Horm Behav 80:47–57. https://doi.org/10.1016/j.yhbeh.2016.01.017
Baier CJ, Katunar MR, Adrover E et al (2012) Gestational restraint stress and the developing dopaminergic system: an overview. Neurotox Res 22:16–32. https://doi.org/10.1007/s12640-011-9305-4
Pallarés ME, Antonelli MC (2017) Prenatal stress and neurodevelopmental plasticity: relevance to psychopathology. In: Adv Exp Med Biol 117–129. https://doi.org/10.1007/978-3-319-62817-2_7
Barros VG, Duhalde-Vega M, Caltana L et al (2006) Astrocyte–neuron vulnerability to prenatal stress in the adult rat brain. J Neurosci Res 83:787–800. https://doi.org/10.1002/jnr.20758
Pallarés ME, Baier CJ, Adrover E et al (2013) Age-dependent effects of prenatal stress on the corticolimbic dopaminergic system development in the rat male offspring. Neurochem Res 38:2323–2335
Monteleone MC, Adrover E, Pallarés ME et al (2014) Prenatal stress changes the glycoprotein gpm6a gene expression and induces epigenetic changes in rat offspring brain. Epigenetics 9:152–160. https://doi.org/10.1007/s12031-018-1101-7
Monteleone MC, Pallarés ME, Billi SC et al (2018) In vivo and in vitro neuronal plasticity modulation by epigenetic regulators. J Mol Neurosci 65:301–311. https://doi.org/10.1007/s12031-018-1101-7
Adrover E, Pallarés ME, Baier CJ et al (2015) Glutamate neurotransmission is affected in prenatally stressed offspring. Neurochem Int 88:73–87. https://doi.org/10.1016/j.neuint.2006.10.009
Pastor V, Pallarés ME, Antonelli MC (2018) Prenatal stress increases adult vulnerability to cocaine reward without affecting pubertal anxiety or novelty response. Behav Brain Res 339:186–194. https://doi.org/10.1016/j.bbr.2017.11.035
Barros VG, Rodriguez P, Martineja ID et al (2006) Prenatal stress and early adoption effects on benzodiazepine receptors and anxiogenic behavior in the adult rat brain. Synapse 61:790–794. https://doi.org/10.1002/syn
Grundwald NJ, Brunton PJ (2015) Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner. Psychoneuroendocrinology 62:204–216. https://doi.org/10.1016/j.psyneuen.2015.08.010
Kertes DA, Kamin HS, Hughes DA et al (2016) Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the Democratic Republic of Congo. Child Dev 87:61–72. https://doi.org/10.1111/cdev.12487
St-Cyr S, Abuaish S, Sivanathan S, McGowan PO (2017) Maternal programming of sex-specific responses to predator odor stress in adult rats. Horm Behav 94:1–12. https://doi.org/10.1016/j.yhbeh.2017.06.005
Maccari S, Krugers HJ, Morley-Fletcher S et al (2014) The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol 26:707–723. https://doi.org/10.1111/jne.12175
Labermaier C, Kohl C, Hartmann J et al (2014) A polymorphism in the Crhr1 gene determines stress vulnerability in male mice. Endocrinology 155:2500–2510. https://doi.org/10.1210/en.2013-1986
Ivy AS, Rex CS, Chen Y et al (2010) Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci 30:13005–13015. https://doi.org/10.1523/JNEUROSCI.1784-10.2010
Xu L, Sun Y, Gao L et al (2014) Prenatal restraint stress is associated with demethylation of corticotrophin releasing hormone (CRH) promoter and enhances CRH transcriptional responses to stress in adolescent rats. Neurochem Res 39:1193–1198. https://doi.org/10.1007/s11064-014-1296-0
Szymańska M, Budziszewska B, Jaworska-Feil L et al (2009) The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and FKBP51 concentration in prenatally stressed rats. Psychoneuroendocrinology 34:822–832. https://doi.org/10.1016/j.psyneuen.2008.12.012
Candemir E, Post A, Dischinger US et al (2019) Limited effects of early life manipulations on sex-specific gene expression and behavior in adulthood. Behav Brain Res 369:111927. https://doi.org/10.1016/j.bbr.2019.111927
Schmidt M, Braun K, Brandwein C et al (2018) Maternal stress during pregnancy induces depressive-like behavior only in female offspring and correlates to their hippocampal Avp and Oxt receptor expression. Behav Brain Res 353:1–10. https://doi.org/10.1016/j.bbr.2018.06.027
Weaver ICG, Cervoni N, Champagne FA et al (2004) Epigenetic programming by maternal behavior. Nat Neurosci. https://doi.org/10.1038/nn1276
Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ (2012) Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos Trans R Soc Lond B Biol Sci 367:2495–2510. https://doi.org/10.1098/rstb.2012.0223
Kundakovic M, Gudsnuk K, Franks B et al (2013) Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1214056110
Bostic JQ, Rubin DH, Prince J, Scholzman S (2005) Treatment of depression in children and adolescents. Handb Interv Work Child Adolesc Prev Treat 11:301–328. https://doi.org/10.1002/9780470753385.ch13
Callaghan B, Meyer H, Opendak M et al (2019) Annual review of clinical psychology using a developmental ecology framework to align fear neurobiology across species. Annu Rev Clin Psychol 15:345–369. https://doi.org/10.1146/annurev-clinpsy-050718
Capone F, Bonsignore LT, Cirulli F (2005) Methods in the analysis of maternal behavior in the rodent. In: Teratology and Developmental Toxicity. p supplement 25–13.9.1–13.9.15. https://doi.org/10.1002/0471140856.tx1309s26
Zhang J, Xue M, Mei Y et al (2020) Co-expression network of mRNAs and lncRNAs regulated by stress-linked behavioral assays. Psychopharmacology 237:571–582. https://doi.org/10.1007/s00213-019-05390-1
Hao Y, Huang W, Nielsen DA, Kosten TA (2011) Litter gender composition and sex affect maternal behavior and DNA methylation levels of the Oprm1 gene in rat offspring. Front Psychiatry 2:12. https://doi.org/10.3389/fpsyt.2011.00021
Murgatroyd CA, Nephew BC (2013) Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 38:219–228. https://doi.org/10.1016/j.psyneuen.2012.05.020
Ivy AS, Brunson KL, Sandman C, Baram TZ (2008) Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154:1132–1142. https://doi.org/10.1016/j.neuroscience.2008.04.019
Grigoryan G, Segal M (2016) Lasting differential effects on plasticity induced by prenatal stress in dorsal and ventral hippocampus. Neural Plast 10. https://doi.org/10.1155/2016/2540462
Viola TW, Wearick-Silva LE, Creutzberg KC et al (2019) Postnatal impoverished housing impairs adolescent risk-assessment and increases risk-taking: a sex-specific effect associated with histone epigenetic regulation of Crfr1 in the medial prefrontal cortex. Psychoneuroendocrinology 99:8–19. https://doi.org/10.1016/j.psyneuen.2018.08.032
Arrant AE, Schramm-Sapyta NL, Kuhn CM (2013) Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res 1:119–127. https://doi.org/10.1016/j.bbr.2013.05.035
Miranda-Morales RS, Pautassi RM (2016) Pharmacological characterization of the nociceptin/orphanin FQ receptor on ethanol-mediated motivational effects in infant and adolescent rats. Behav Brain Res 1:88–96. https://doi.org/10.1016/j.bbr.2015.04.016
Slattery DA, Cryan JF (2012) Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7:1009–1014. https://doi.org/10.1038/nprot.2012.044
Commons KG, Cholanians AB, Babb JA, Ehlinger DG (2017) The rodent forced swim test measures stress-coping strategy, not depression-like behavior HHS public access. ACS Chem Neurosci 8:955–960. https://doi.org/10.1021/acschemneuro.7b00042
Zubiría MG, Alzamendi A, Moreno G et al (2016) Relationship between the balance of hypertrophic/hyperplastic adipose tissue expansion and the metabolic profile in a high glucocorticoids model. Nutrients 8:16. https://doi.org/10.3390/nu8070410
Ruijter JM, Ramakers C, Hoogaars WMH et al (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:12. https://doi.org/10.1093/nar/gkp045
Gentle A, Anastasopoulus F, McBrien NA (2001) High-resolution semi-quantitative real-time PCR without the use of a standard curve. Biotechniques 31:502–507. https://doi.org/10.2144/01313st03
Vandesompele J, De Preter K, Pattyn F et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12. https://doi.org/10.1186/gb-2002-3-7-research0034
Monteleone MC, Pallarés ME, Billi SC et al (2018) In vivo and in vitro neuronal plasticity modulation by epigenetic regulators. J Mol Neurosci 65:301–311. https://doi.org/10.1007/s12031-018-1101-7
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:1–12. https://doi.org/10.3389/fpsyg.2013.00863
Bourke CH, Raees MQ, Malviya S et al (2013) Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology 38:84–93. https://doi.org/10.1016/j.psyneuen.2012.05.001
Green MR, Nottrodt RE, Simone JJ, McCormick CM (2016) Glucocorticoid receptor translocation and expression of relevant genes in the hippocampus of adolescent and adult male rats. Psychoneuroendocrinology 73:32–41. https://doi.org/10.1016/j.psyneuen.2016.07.210
Foltran RB, Diaz SL (2016) BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem 138:204–221. https://doi.org/10.1111/jnc.13658
Boersma GJ, Lee RS, Cordner ZA et al (2013) Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats. Epigenetics 9:437–447. https://doi.org/10.4161/epi.27558
Neeley EW, Berger R, Koenig JI, Leonard S (2011) Prenatal stress differentially alters brain-derived neurotrophic factor expression and signaling across rat strains. Neuroscience 187:24–35. https://doi.org/10.1016/j.neuroscience.2011.03.065
Hillerer KM, Neumann ID, Slattery DA (2012) From stress to postpartum mood and anxiety disorders: how chronic peripartum stress can impair maternal adaptations. Neuroendocrinology 95:22–38. https://doi.org/10.1159/000330445
Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS (2014) On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol 35:245–251. https://doi.org/10.1016/j.yfrne.2013.11.002
Patin V, Lordi B, Vincent A et al (2002) E ffects of prenatal stress on maternal behavior in the rat. Dev Brain Res 139:1–8. https://doi.org/10.1016/s0165-3806(02)00491-1
Fodor A, Klausz B, Pintér O et al (2012) Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Horm Behav 62:539–551. https://doi.org/10.1016/j.yhbeh.2012.09.003
Meek LR, Dittel PL, Sheehan MC et al (2001) Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav 72:473–479. https://doi.org/10.1016/s0031-9384(00)00431-5
Hauser J, Feldon J, Pryce CR (2009) Direct and dam-mediated effects of prenatal dexamethasone on emotionality, cognition and HPA axis in adult Wistar rats. Horm Behav 56:364–375. https://doi.org/10.1016/j.yhbeh.2009.07.003
Molet J, Maras PM, Avishai-Eliner S, Baram TZ (2015) Naturalistic rodent models of chronic early-life stress. Dev Psychobiol 56(8):1675–88. https://doi.org/10.1002/dev.21230
Stöhr T, Wermeling DS, Szuran T et al (1998) Differential effects of prenatal stress in two inbred strains of rats. Pharmacol Biochem Behav 59:799–805. https://doi.org/10.1016/S0091-3057(97)00541-8
Schroeder M, Sultany T, Weller A (2013) Prenatal stress effects on emotion regulation differ by genotype and sex in prepubertal rats. Dev Psychobiol 55:176–192. https://doi.org/10.1002/dev.21010
Nederhof E, Schmidt MV (2012) Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106:691–700. https://doi.org/10.1016/j.physbeh.2011.12.008
Rozeboom AM, Akil H, Seasholtz AF, Mcewen BS (2007) Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. PNAS 104:4688–4693. https://doi.org/10.1073/pnas.0606067104
ter Heegde F, De Rijk RH, Vinkers CH (2015) The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 52:92–110. https://doi.org/10.1016/j.psyneuen.2014.10.022
Chen Y, Brunson KL, Adelmann G et al (2004) Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience 126:533–540. https://doi.org/10.1016/j.neuroscience.2004.03.036
Stern CM (2011) Corticotropin-releasing factor in the hippocampus: eustress or distress? J Neurosci 31:1935–1936. https://doi.org/10.1523/JNEUROSCI.5611-10.2011
Wang X, Meng F Sen, Liu ZY, et al (2013) Gestational hypoxia induces sex-differential methylation of Crhr1 linked to anxiety-like behavior. Mol Neurobiol 48:544–55. https://doi.org/10.1007/s12035-013-8444-4
Xu Y-J, Sheng H, Wu T-W et al (2018) CRH/CRHR1 mediates prenatal synthetic glucocorticoid programming of depression-like behavior across 2 generations. FASEB J 32:12. https://doi.org/10.1096/fj.201700948RR
Bennett MR, Lagopoulos J (2014) Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol 112:80–99. https://doi.org/10.1016/j.pneurobio.2013.10.005
Xiao Z, Wanhong L, Gao K et al (2011) Interaction between CRHR1 and BDNF genes increases the risk of recurrent major depressive disorder in chinese population. PLoS ONE 6:5. https://doi.org/10.1371/journal.pone.0028733
Papale LA, Madrid A, Li S, Alisch RS (2017) Early-life stress links 5-hydroxymethylcytosine to anxiety-related behaviors. Epigenetics 12:264–276. https://doi.org/10.1080/15592294.2017.1285986
Khare T, Pai S, Koncevicius K et al (2012) 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary. Nat Struct Mol Biol 19:1037–1043. https://doi.org/10.1038/nsmb.2372
Acknowledgements
The authors greatly appreciate the technical assistance of Ms. Susana Buglione, Ms. Mercedes Imsem, and Dr. Silvia Billi for technical assistance. M.E.P., M.C.M., V.P., A.A., M.A.B., and M.C.A. are researchers from CONICET. J.G.B. is an undergraduate student from Universidad de Buenos Aires.
Funding
This research was financially supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT 2015/BID 2469 to MEP), by CONICET (grant PIP 0249/12 and 0717/2015 to MCA) and by the UNSAM (Grants 2014- 2015 to MAB).
Author information
Authors and Affiliations
Contributions
M.E.P. designed, performed, analyzed all the experiments, and wrote the manuscript. M.C.M. helped with the development of real-time qPCR and chromatin remodeling experiments. V.P. helped with the development of prenatal stress protocol. J.G.B. analyzed behavioral data from maternal behavior. A.A. performed RIA analyses to detect corticosterone levels in serum. M.A.B. supervised the design, development, and analyses of molecular experiments. M.C.A. supervised the whole study design, development, and data analyses. M.C.A. also contributed to the final version of the paper. All authors made manuscript revisions and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
This research was performed in accordance with the standards for the care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals (NIG Publications No. 8023, revised 1978). All protocols were approved by the Institutional Animal Care and Use Committee (CICUAL), Facultad de Medicina (School of Medicine), Universidad de Buenos Aires.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pallarés, M.E., Monteleone, M.C., Pastor, V. et al. Early-Life Stress Reprograms Stress-Coping Abilities in Male and Female Juvenile Rats. Mol Neurobiol 58, 5837–5856 (2021). https://doi.org/10.1007/s12035-021-02527-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02527-2