Abstract
There is a worldwide effort to increase the efficiency of potato (Solanum tuberosum L.) cultivar development by using inbred diploid breeding lines. This activity is impeded by the cost and effort required to produce dihaploids from cultivated tetraploid potatoes. We developed a high throughput dihaploid production method based on the 60-year-old method of Peloquin and Hougas. Red Norland inflorescences from commercial fields were transferred to greenhouses. As buds developed, pollen from the dihaploid inducer IVP 101 was applied systematically to thousands of stigmas per trial. Berries were harvested 21 days after pollination. Seeds of putative dihaploids lacking a seed spot marker were retained and ploidy was confirmed using flow cytometry. We recovered 23 dihaploids from 21,651 pollinations. This is a promising method for systematically carrying out thousands of pollinations since the cost of field-grown flowers is dramatically less than that of greenhouse-grown flowers.
Resumen
Existe un esfuerzo mundial para aumentar la eficiencia del desarrollo de variedades de papa (Solanum tuberosum) mediante el uso de líneas de reproducción diploides endogámicas. Esta actividad se ve obstaculizada por el costo y el esfuerzo requeridos para producir dihaploides a partir de papas tetraploides cultivadas. Desarrollamos un método de producción de dihaploides de alto rendimiento basado en el método de 60 años de Peloquin y Hougas. Las inflorescencias de Red Norland de los campos comerciales se transfirieron a invernaderos. A medida que se desarrollaron las yemas, el polen del inductor dihaploide IVP 101 se aplicó sistemáticamente a miles de estigmas por ensayo. Se cosecharon las bayas 21 días después de la polinización. Las semillas de los supuestos dihaploides que carecían un marcador de la semilla se conservaron y se confirmó la ploidía usando citometría de flujo. Recuperamos 23 dihaploides de 21.651 polinizaciones. Este es un método prometedor para llevar a cabo sistemáticamente miles de polinizaciones, ya que el costo de las flores cultivadas en el campo es dramáticamente menor que el de las flores cultivadas en invernadero.
Similar content being viewed by others
Introduction
Genetic improvement in cultivated potato is inefficient owing to complications that arise from the tetraploid nature of current cultivars (Lindhout et al. 2011; Jansky et al. 2016). As an alternative to tetraploid potato breeding, we and others are exploring a transition to a simplified diploid breeding system (Lindhout et al. 2011; Jansky et al. 2016; Stokstad 2019). With a diploid system, inbred lines can be generated and crossed, with predictable outcomes, to produce hybrid potato varieties. Backcrossing can add traits to inbred parental lines, with subsequent crosses producing hybrids carrying the trait. We are calling this new breeding system Potato 2.0. Two major developments must occur during the transition from a tetraploid to a diploid crop. First, we must capture in diploid individuals the beneficial alleles present in tetraploid cultivars. Second, germplasm with commercial potential must be generated from those individuals through breeding. This paper focuses on the first goal – generating diploid individuals that contain the genetic information present in tetraploid cultivars.
An effective technique for ploidy reduction in potato has been available for over 60 years (Hougas and Peloquin 1957; Hougas and Peloquin 1958; Hougas et al. 1958). When tetraploid clones are crossed to certain diploid pollen donors, dihaploid offspring are produced at a very low frequency. It is unclear whether this process is due to parthenogenesis or chromosome elimination (Montelongo-Escobedo and Rowe 1969; Clulow et al. 1991; Peloquin et al. 1996; Samitsu and Hosaka 2002; Ercolano et al. 2004; Amundson et al. 2020). In either case, the result is essentially the same. Offspring are products of meiosis and, as such, are genetically variable and contain two of the four genetic complements of the tetraploid parent. The diploid pollen donor does not appear to contribute substantially to the genetic control of dihaploid offspring (Samitsu and Hosaka 2002; Amundson et al. 2020).
The term haploid refers to a sporophyte with the gametophytic chromosome number (Dwivedi et al. 2015). While this term is technically correct and has been used extensively in past potato literature, we have chosen to use the more descriptive term dihaploid. It emphasizes the diploid nature of haploids derived from tetraploid individuals.
Fertile dihaploids cross readily with many diploid wild potato species and have been used extensively in germplasm enhancement efforts (Hanneman Jr. and Peloquin 1969; Hermundstad and Peloquin 1985; Peloquin et al. 1989; Jansky et al. 1990; Ortiz et al. 2009). When a large number of dihaploids are available, they are also useful for genetic mapping, since dihaploids from a clone represent a collection of egg cell genotypes (Kotch et al. 1992). Hougas and Peloquin (1958) suggested the use of dihaploids in potato breeding could allow breeders to move towards homozygosity and more readily elucidate the genetic basis of agronomic traits. Dihaploids are valuable for revealing both the positive and negative genetic variation hidden in their tetraploid parents (Peloquin and Hougas 1960; Kotch 1987). For example, dihaploids with levels of disease resistance higher than their tetraploid parents have been identified (Cipar and Lawrence 1972; Jansky et al. 2003; Ercolano et al. 2004).
Low fertility is common in potato cultivars (Bethke and Jansky 2021 and references therein) and extraction of a dihaploid from a tetraploid results in a level of inbreeding similar to three generations of self-pollinating an autotetraploid (Hougas and Peloquin 1958). Genetic load is high in clonally propagated crops, including potato (Manrique-Carpintero et al. 2019; Zhang et al. 2019). An inverse relationship likely exists between genetic load in a tetraploid clone and the ability to extract dihaploids from it (Hougas et al. 1964). As a result of poor fertility in tetraploid parents and inbreeding depression in their resulting diploid offspring, the production of functional flowers with viable pollen is rare in dihaploids (Peloquin and Hougas 1960). Thus, it is necessary to produce a large number of dihaploids in order to identify fertile clones that carry genes of interest for potato breeding.
Dihaploid induction efficiency is typically very low. Pollinations produce few berries and low numbers of seeds per berry (Hougas et al. 1964; Hermsen and Verdenius 1973; Hutten et al. 1994). To capture the beneficial alleles found in a single tetraploid clone will require the production of several dihaploids from that clone. Thus, a strategy for rapidly and inexpensively pollinating flowers with pollen from dihaploid inducers on a large scale is desirable.
Dihaploid extraction is typically carried out in greenhouses using tetraploid cultivars grown in pots. Greenhouse production is expensive relative to field production, and greenhouses impose strict space limitations. An alternative method was described by Peloquin and Hougas in the late 1950s. In that method, flower-bearing stems from “decapitated” potato plants were collected from field plots and brought back to greenhouses for pollination (McLean and Stevenson 1952; Peloquin and Hougas 1959). Although this approach made it possible to conduct large-scale dihaploid extractions, many steps in the process remained costly, tedious or inefficient. In particular, field plots are unnecessarily expensive when the plants are grown only to produce flowers. Seedlings were scored for attributes associated with diploid potatoes and this necessitated growing seedlings, most of which had no value, in greenhouses. Also, screens for dihaploids based on pigmented markers were not confirmed by complementary methods.
Commercial potato fields contain an abundance of potato flowers that come into bloom in near synchrony. Potato growers have no use for these flowers. We therefore developed workflows in which flowers produced in commercial potato fields are the foundation of dihaploid extraction efforts. Further refinements in the techniques for producing fruit and scoring dihaploids were introduced as our workflow became more mature.
Materials and Methods
2017 Season, Wisconsin
Certified seed tubers of Solanum tuberosum cv. Red Norland were planted at Coloma Farms, Inc. (Coloma, WI 54930) on April 11, 2017. A total of 408 shoots that had inflorescences in which the first flower bud was open or enlarged and soon to open were collected on June 11 and 12. Most of the material was collected on June 12 by two people working 2 h. Cut shoots were 30 to 40 cm long and stem bases were placed in plastic buckets containing water immediately after cutting. Shoots collected June 11 were held overnight at 20 °C. Leaves 10 to 15 cm or more below the shoot apex were removed from all shoots on June 12. Three shoots were placed in each 0.6 L glass bottle containing tap water (Fig. 1a). Open flowers and small flower buds in which the corolla did not extend beyond the calyx were removed (Fig. 1b). The remaining 1200 flower buds had corolla removed and were emasculated (Fig. 1c). Pollen from Solanum tuberosum Phureja Group IVP 101 (IVP 101) that had been collected previously and stored in gelatin capsules at −20 °C was applied to each stigma. Approximately 10 flowers were pollinated per bottle. Seventeen heavy duty plastic horticultural flats, 52 cm × 37 cm, containing eight bottles each were placed in a 3.65 m × 2.64 m walk-in growth chamber at 20 °C with 24 h photoperiod and photon flux density of 400 μmol m−2 s−1 in the University of Wisconsin-Madison Biotron Laboratory (Madison, WI 53706). Leaf removal, flower bud preparation and pollinations were completed in three hours by a team of seven people. After 24 h at 20 °C, the temperature was lowered to 18 °C to better promote berry development. When bottles required additional water, they were thoroughly flushed before filling to reduce microbial growth. Colorado potato beetle (Leptinotarsa decemlineata) control was done manually. Berries were harvested 21 days after pollination (Fig. 1d). Berries were held at room temperature for an additional 21 days before seed extraction.
Red Norland shoots prepared for crossing with IVP 101 and fruit development after 21 days. (a) Shoot in 0.6 L glass bottle after trimming excess foliage. (b) Close up of an inflorescence. White asterisks indicate open flowers and small buds that were removed prior to emasculation. (c) Inflorescence after emasculation. (d) Shoot bearing fruit 21 days after pollination with IVP 101
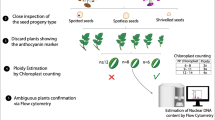
Seeds were removed from berries and the number of seeds in each berry was noted. Seeds were dried on paper towels at room temperature. Each seed was viewed with a dissecting microscope for the presence of a seed spot marker (Hermsen and Verdenius 1973). Seeds bearing the seed spot marker are likely hybrids between IVP 101 and Red Norland, and these seeds were discarded (Fig. 2). Seeds without the marker were soaked overnight in 3.9 mM gibberellic acid potassium salt (Sigma-Aldrich, St. Louis, MO) prepared using reverse-osmosis water. Seeds were then sown in potting soil in a greenhouse.
Epidermal peels were made from fully expanded leaves of putative dihaploids and the number of chloroplasts per guard cell was determined by viewing peels in water using a compound microscope with a 40x objective and 10x eyepiece (Leica ICC50 HD, Leica Microsystems GmbH Wetzlar, Germany). Typically, chloroplast number in two guard cells from different stomata was determined for each of four epidermal peels per individual. Plants having six or fewer chloroplasts per guard cell on average were provisionally classified as dihaploids (Ordoñez et al. 2017).
Nearly open flower buds of putative dihaploids were selected, emasculated and crossed with diploid pollen. Every putative dihaploid that produced flowers was crossed with US-W4 pollen. US-W4 is a clonally maintained dihaploid from the tetraploid clone Minnesota 20–20-34 (2n = 4x = 48). Pollen from self-compatible mcd1, derived from a self-compatible Solanum microdontom of unknown origin (2n = 2x = 24), was also used for all putative dihaploids which flowered except for clones 9 and 12 which produced very few flowers.
Ploidy was confirmed by flow cytometry using a CyFlow Ploidy Analyzer (Systemex, Lincolnshire, IL). IVP 101 and Red Norland grown in a growth chamber at 20 °C with a 16-h photoperiod were used as diploid and tetraploid controls, respectively. Small leaflets from each individual were collected and stored at 4 °C in the dark overnight. For each sample, approximately 50 mg of leaf material was placed in a plastic dish with 1 mL CyStain® PI Absolute P nuclei extraction buffer (Systemex). Leaf material was sliced into <0.5 mm pieces with a sharp blade, filtered into a tube using CellTrics® filters (Systemex) and nuclei in the filtrate were stained with 10 mL of CyStain® UV Precise P (Systemex). Sample tubes were inverted twice and placed on ice for five minutes before analysis with the flow cytometer. The nuclei extraction buffer and staining buffer were stored in the dark on ice during sample processing. A cleaning solution and decontamination solution were used between samples.
We observed a large number of parthenocarpic berries in this study, and we investigated the possibility that the cut-stem technique induced parthenocarpy. Open-pollinated berries were harvested from Coloma Farms, Inc. after vines had senesced in late July 2017. Berries were examined individually and the number of seeds in each was counted.
2018 Season, Wisconsin
The methods used in 2018 were similar to those used 2017. Briefly, certified Red Norland seed tubers were planted and fields maintained by Alsum Farm and Produce, Inc. (Friesland, WI 53935). Two people spent 3.5 h collecting shoots on June 14, 2018. Three people working four hours removed lower leaves from shoots and placed three shoots each into 0.6 L glass bottles and transferred the bottles to air-conditioned greenhouses at 20–22 °C. Five people working for 28 h total emasculated flowers the next day. Previously collected IVP 101 pollen was applied to the stigmas of 7800 flowers by one person over a period of seven hours using an artist paintbrush on June 16, 2018. Colorado potato beetles (Leptinotarsa decemlineata) were removed manually. Berries were harvested 28 days after pollination and held at room temperature for an additional 21 days before extraction.
Every putative dihaploid that produced flowers in this trial was crossed with pollen from M6, a highly inbred clone of Solanum chacoense (2n = 2x = 24) (Jansky et al. 2014).
2018 Season, Minnesota
Shoots of Red Norland grown from certified seed were collected before noon from Edling Farms, Inc. (Clear Lake, MN 55319) on June 22; Del Hayes and Sons Potato Warehouse, Inc. (Big Lake, MN 55309) on June 22 and 25; Gray Potato Farm, Inc. (Clear Lake, MN 55319) on June 27; and University of Minnesota-Sand Plains Research Farm, (Becker, MN 55308) on July 6. Improvements to the collecting technique were made over the shoot collection period. Berry formation was found to be best on 45 cm shoots each bearing an inflorescence with open or nearly open flowers and one subtending leaf. Shoots were collected into buckets containing an aqueous solution of 2 μg mL−1 CarboLoad (Advanced Nutrients, Abbotsford, B.C. V2T 6H1 Canada) and 10,000 μg mL−1 Chrysal Professional 3 (Chrysta, Miami, FL). Shoots were transported to the University of Minnesota-St. Paul where senescing flowers and small flower buds were removed. Remaining flowers were not emasculated and were pollinated with previously-collected pollen from IVP 101 that had been stored at −20 °C. Shoots were recut and placed in plastic tubs covered with metal mesh to keep stems upright and separate. Each tub contained several cm of fresh collection solution supplemented with household bleach to a final concentration of 776.0 μM sodium hypochlorite. Stems were recut and the solution was replaced weekly. Shoots were placed near a window in a room where the lights remained on at all times. Colorado potato beetles were removed manually.
Berries were collected 3 to 4 weeks after pollination or when they fell off the stems. The ripening process took 1 to 3 weeks and berries were considered to be ripe when they were soft to the touch. Seeds were extracted from ripe berries by squeezing under water and dried at room temperature. After discarding seeds with the embryo spot marker, the remaining dry seeds were surface sterilized by soaking in 77.6 mM sodium hypochlorite. To reduce dormancy, seeds were soaked in autoclaved 3.9 mM gibberellic acid (Phyto Tech labs, Lenexa, KS 66215) for 24 h prior to sowing on sterile Murashige and Skoog (MS) media. Seeds were maintained in the dark until germination. After germination, seedlings on MS media were placed on shelves fitted with grow lights. Ploidy of each individual seedling was confirmed using flow cytometry as described above. Because samples were not pre-screened using chloroplast counting, we expected the majority to be tetraploid. Therefore, samples were bulked in groups of 5 (10 mg of tissue per plant) for flow cytometry. If a diploid peak was visible, each individual in the bulked sample was tested separately to identify the dihaploid.
2019 Season, Minnesota
The methods were similar to those used in 2018. Flowers were collected on June 28 at Wingard Farms, Elk River, MN, 55330; on July 3 and 6 at Ewing Farms Inc., Big Lake MN, 55309; and on July 10 at the University of Minnesota-Sand Plains Research Farm, Becker, MN 55308. Pollen was applied to stigmas using a cotton-tipped applicator.
Results
Shoots collected in Wisconsin in 2017 and 2018 and Minnesota in 2018 and 2019 did not show significant rot after three weeks in tap water (Fig. 1d) or in tap water supplemented with nutrients and a biocide. In general, stems collected early in the growing season were less likely to foster microbial growth than those collected later in the season.
Findings related to seeds per berry and assays used to identify dihaploid progeny are described in detail for the Wisconsin trial in 2017 and summarized briefly for the other trials. For the Wisconsin trial in 2017, 534 berries were processed individually owing to concerns that inadvertent self-pollination may have occurred if anther dehiscence occurred before buds opened. Since self-pollinated flowers are expected to produce berries with more seeds than those from crosses to the IVP 101 pollinator, we discarded seeds from berries containing more than 10 seeds. Only one berry had more than 10 seeds; it had 16 seeds (Table 1). The majority of berries, 378 of 534 berries, were parthenocarpic. Of the berries carrying seeds, 82 berries had one, 39 had two, 15 had three, 13 had four, 3 had five, 2 had six and 1 had seven seeds for a total of 171 seeds from 533 fruit.
Ninety seeds (52.6%) contained the seed spot marker, indicative of a hybrid with the pollinator IVP 101 (Fig. 2). These seeds were discarded. The remaining 81 seeds were sown; 19 seedlings emerged, 12 seedlings grew into mature plants and 7 plants flowered. Seven of the 12 plants were identified as putative dihaploids based on an average of 6 or fewer chloroplasts per guard cell (Table 2). Three of these flowered and were confirmed as dihaploids using flow cytometry. One plant with an average of 7 chloroplasts per guard cell was also found to be diploid using flow cytometry. This plant also produced flowers. An additional 4 putative dihaploids based on guard cell chloroplast numbers were lost in an organizational error and not evaluated with flow cytometry (Table 2). Lines which flowered were pollinated by various diploid clones that rarely if ever produce 2n pollen. Only a few flowers were produced. Thus, it was difficult to confirm ploidy with these pollination data. However, clone number 5 produced one berry when crossed with self-compatible mcd1 and produced 30 seeds, providing further evidence that it is a dihaploid. One of the flow-cytometry-verified dihaploid seedlings from the 2018 Wisconsin trials flowered and was crossed with M6. This plant produced two fruit containing 46 seeds. The 22 seedlings recovered segregated for phenotypes of the parents.
Figure 3 shows four dihaploid plants produced from Red Norland. All four plants, clones 1, 3, 5 and 12, were similar in appearance. Plants were compact with compound leaves. The dihaploid plants produced in the 2018 Wisconsin trial had a wider morphological range than those produced in 2017. Two of these were vigorous, upright flowering plants. One individual was vigorous but more compact and did not flower. Three individuals were diminutive and did not flower.
Tuber periderm color of the four dihaploids produced in 2017 ranged from red to white (Fig. 4). Clone 1 produced potatoes with dark red skin (Fig. 4a). The tubers produced by clones 3 and 5 had red pigmented eyes but very little red coloration in the periderm (Fig. 4b and c). The periderm of clone 12 was devoid of red pigmentation (Fig. 4d). All six of the dihaploids produced in 2018 in Wisconsin had red or light red pigmented periderm (data not shown).
Findings from each of the four trials are presented in Table 3 and summarily show that dihaploids were produced from Red Norland at a low frequency. The number of berries needed for the production of one dihaploid from Red Norland in these four trials ranged from 77 to 241. Likewise, the number of pollinations required for one dihaploid ranged from 300 to 1323. The overall recovery rate of dihaploids from Red Norland was less than one per 100 berries or less than one per 900 crosses. Substantial variation was observed in the number of unspotted seeds per berry. Parthenocarpy was common. The majority of berries produced during 2018 in Wisconsin and at least 44% of the berries produced in Minnesota across both years bore no seeds. The germination rate of seeds lacking the seed spot marker was quite variable, ranging from 23.4% in the first Wisconsin trial to 46% in the first Minnesota trial.
The dihaploid extraction experiments with Red Norland raised questions about the fecundity of that variety. One question related to the ability of Red Norland to form and retain berries with few or no seeds. We collected 226 open-pollinated Red Norland berries from a commercial potato farm and found that parthenocarpy was prevalent, with 71.5% of berries containing no seeds (Table 4). This is nearly identical to the 70.7% of berries with no seeds observed in the 2017 Wisconsin trial (Table 1). Ninety-six percent of berries from the production field had twenty or fewer seeds (Table 4). The two berries with the largest number of seeds had 53 and 61 seeds.
The time input for shoot collection, flower preparation and emasculation varied from 6.3 h to 9 h for every dihaploid produced in the 2017 and 2018 Wisconsin trials respectively and 6.6 h per dihaploid in the Minnesota trial where flowers were not emasculated However, the number of pollination events per hour invested increased three-fold from 48 h−1 to 144 h−1 in the 2017 and 2018 WI trials, respectively.
Discussion
Producing diploid inbred lines that can be crossed to produce hybrid potato varieties is a goal of the Potato 2.0 breeding system. Prior research has shown that dihaploids are produced from tetraplaoid potato at a very low frequency. In light of these considerations, we developed a streamlined, high throughput system for generating dihaploids from tetraploid potato. Using this system, we produced Red Norland dihaploids at facilities at the University of Minnesota and the University of Wisconsin in each of two years (Table 3). The fundamental process used at both locations was similar. We relied on commercial potato fields as a source of flowering shoots. Ample IVP 101 pollen was collected in advance and stored frozen. Labor-intensive, time-critical portions of the process were restricted to a few days each year. Pollination and berry development took place in favorable environments under our control. Ploidy was confirmed by flow cytometry to accurately identify dihaploids and discard unwanted progeny. Moreover, in both 2018 and 2019, collections were made in Minnesota from several growers, and this allowed us to broaden our collection time window owing to differences in plant development between locations.
Additional aspects of our system contribute to its high throughput capacity. Emasculation is a time-consuming process that may damage the stigma. Spontaneous self-pollination is rare in many potato varieties as long as they are not shaken (Bienz 1958). We did not emasculate flowers before pollinating in Minnesota trials. No problems were observed in taking this time-saving step. Emasculation may be necessary, however, when working with highly self-compatible potato lines. We harvested each berry individually, as described above. Berries with high seed set were discarded since they may have resulted from self-pollination. We also pollinated with a soft-bristled artist brush or cotton-tipped applicator. Since IVP 101 was the only pollen source, the brush or applicator could be loaded with pollen and used continuously with no need to clean it. Since we collected a large number of shoots, we typically filled an entire greenhouse or growing space with shoots from only one cultivar. This saved time as we did not need to label containers. In addition, any berries we found in the greenhouse, even those that fell off plants, were assumed to have been produced from the cultivar in that greenhouse.
Pollinating flowers on shoots removed from the mother plant has been reported to improve pollination success (Peloquin and Hougas 1959), perhaps because developing berries do not need to compete with developing tubers for nutrients (McLean and Stevenson 1952). This “decapitation” method significantly increases the number of berries and seeds produced from difficult matings. In addition, it can be implemented on a large scale, and pollinations can be carried out under controlled conditions in a relatively small space. Another option to avoid competition from the tuber sink is to graft potato stems onto tomato rootstocks (Hutten et al. 1994; Panahandeh 2010). However, this is a labor-intensive process.
Dihaploid extraction efficiency is influenced by the genotypes of the parents. In potato dihaploid literature, it is common to report the number of dihaploids per 100 berries. This avoids outside influences such as the effect of environment on berry set (which would impact the number of dihaploids per pollination) and the production of selfed and hybrid seeds (which would impact the number of dihaploids per 100 seeds produced) (Liu and Douches 1993). The tetraploid female parent influences dihaploid induction success (Hougas et al. 1964; Hermsen and Verdenius 1973; Panahandeh 2010). In studies comparing dihaploid induction across tetraploid female parents, the maximum number of dihaploids per 100 berries was 4.6 in Merrimack, (Hougas et al. 1964), 6.2 in Atlantic (Liu and Douches 1993), 10.7 in Picasso (Panahandeh 2010), 20.2 in Atlantic (Kotch and Peloquin 1987), 25 in Jana (Dolničar and Bohanec 2000), and 25.6 in Merrimack (Peloquin et al. 1996). To our knowledge, data for Red Norland have not been published. Our overall rate for Red Norland was less than one dihaploid per 100 berries. Parthenocarpy was common in berries produced by crossing Red Norland with the IVP 101 pollinator (70.8%) and in open-pollinated berries (71.5%) (Tables 1 and 3). Much higher numbers of dihaploids per 100 berries, up to 463, have been reported in breeding lines (Hermsen and Verdenius 1973; Hanneman Jr. and Ruhde 1978; Hutten et al. 1994).
Dihaploid extraction for Potato 2.0 is focusing on tetraploid clones likely to contribute valuable traits for the development of diploid cultivars. Our strategy of extracting dihaploids from commercial potato fields ensures that we are concentrating our efforts on germplasm relevant to modern potato production. Unfortunately, as we see with Red Norland, induction frequency in important cultivars may be low. One way to address this low efficiency problem is to use a high-throughput technique to scale up the number of pollinations made per cultivar. The time needed for collecting shoots, preparing shoots, emasculating flowers and pollinating in the 2017 Wisconsin trial was 48 pollinations for each hour of labor invested. An increase in efficiency to 144 pollinations h−1 in the 2018 Wisconsin trial can be attributed primarily to time saved by using an artist brush for pollination. Foregoing emasculation seems justified for cultivars that produce a low number of seeds as a result of self pollination. Eliminating the emasculation step has the potential to reduce pollination time but may add time spent evaluating ploidy.
Since the initial reports of Peloquin and Hougas, selection for improved pollen donor efficiency has been carried out by focusing on high male fertility, homozygosity for the seed spot marker, and the ability to induce a relatively high number of dihaploids (Hermsen and Verdenius 1973). Three pollen parents for dihaploid production are available through the U.S. Potato Genebank (Phureja 1.22, IVP 48 and IVP 101). We chose IVP 101 because it produces abundant pollen, it has a low frequency of 2n pollen production, and the seed spot marker (Fig. 2) is highly penetrant in hybrid offspring. Having a ready supply of pollen on hand was a key feature of our high throughput system. We used an artist paintbrush dipped in pollen to pollinate 7800 pistils in 7 h during the 2018 WI trials. To make sure we had an abundance of pollen on hand for the large number of pollinations needed for this project, we maintained IVP 101 plants throughout the winter and collected pollen over the course of several months. The pollen was desiccated and frozen at −20 °C until it was used in June. Viability of S. phureja pollen has been reported to decrease during cold storage for more than one year (De Maine 1977; De Maine 1988), but shorter-term storage of potato pollen in a freezer does not appear to affect viability (King 1955; Howard 1958; Blomquist and Lauer 1962).
Ploidy in potato can be determined through several techniques and has recently been reviewed (Alsahlany et al. 2019; Kramer and Bamberg 2019). In our research, ploidy of offspring was assessed using guard cell choloroplast counts, flow cytometry, and crossing as females to diploid clones. Flow cytometry provides a direct measurement of ploidy but requires specialized equipment and per assay costs are relatively high. We used two prescreening methods to reduce this cost. The first was to pool flow cytometry samples in groups of 5 for initial screens. The second prescreening method, used in both Wisconsin trials, was to determine the number of chloroplasts per guard cell. This later approach has the advantage of requiring only a compound microscope to do the assay. Ordoñez et al. (2017) published estimates of 6 to 8 chloroplasts per guard cell for dihaploids and 12 to 14 chloroplasts per guard cell for tetraploids. Rasmussen and Rasmussen (1995) noted 5.7 to 6.3 chloroplasts per guard cell for dihaploids and 9.0 to 12.6 for tetraploid Solanum tuberosum, indicating that variability does exist for guard cell chloroplast numbers in both dihaploids and tetraploids. For instance, chloroplast number per cell in potato is known to vary with growing conditions (Singsit and Veilleux 1991). The number of chloroplasts per guard cell in our dihaploid material is roughly one half the number of guard cell chloroplasts of the tetraploid parent.
Crossing plants to diploid parents, especially those that do not readily produce 2n pollen, provides confirmatory evidence that a clone is a dihaploid. However, this approach may fail to identify dihaploids when female fertility is low and few fruit are produced. We observed low female fertility in all of our Red Norland dihaploids. Genotyping with single nucleotide polymorphism (SNP) markers is an alternative method for identifying dihaploids lines, but was not used in this study. SNP genotyping has the additional benefit of identifying aneuploids.
The effect of environment on dihaploid induction success cannot be neglected. Liu and Douches (1993) observed that success in a greenhouse varies across years and seasons. Environment may influence the number of flowers produced and the fertility of those flowers. A limitation of our method is that we cannot control the growth environment of the female parents during flower development. As the season progresses, plants are more likely to experience warm conditions that can reduce fertility and berry production (Clark 1935; Edmundson 1939; Henderson and LeClerg 1943; Bienz 1958). Female fertility declined dramatically in field-grown plants when the average temperature exceeded 21 °C for several days during flower development (Arnason 1943). In our 2017 trial, we had an unusually warm spring. Three of the seven days prior to stem collection had a warmer average temperature than 21 °C. On the day inflourescences were collected, the average temperature was 26 °C and the high on that day was 32 °C. The influence of pre- and post-pollination environments on pollination success is an important topic for additional research.
Sterility in dihaploids is expected as a consequence of inbreeding depression. It is interesting to note that the first (US-W1) and fourth (US-W4) dihaploid generated by Hougas and Peloquin were male fertile (Peloquin and Hougas 1958; Peloquin and Hougas 1960). Experience gained since then has revealed that male fertility is rare. Female fertility can also be problematic for breeding. In one study, bulk pollen applied to 800 diploid clones yielded berries on 118 (15%) of them (Sanford and Hanneman 1982). Fertility problems are likely due to the expression of deleterious recessive genes rather than a lack of chromosome pairing (Yeh et al. 1964). Low female fertility was observed in some of the Red Norland dihaploid offspring generated in this study. For example, flow-cytometry-confirmed clones 1, 3, and 12, which were generated in 2017, did not produce seeds when crossed as a female to diploid lines (Table 2). One of the two dihaploids which produced flowers in the 2018 WI trial likewise did not produce seed when pollinated with M6, which is highly fertile as a male. None of the dihaploid clones we generated have been found to be male fertile. Another consequence of inbreeding depression may be poor seed germination and seedling vigor. Low rates of seed germination have been reported in populations of dihaploids (Caligari et al. 1988; Liu and Douches 1993).
Unresolved technical issues which were not addressed in this work include: A) the value of placing cut stems in water vs a solution containing floral preservative, B) appropriate light source and intensity during fruit development and C) advantages or disadvantages of various fruit ripening methods. We can only speculate on these issues until additional research addresses these important questions.
References
Alsahlany, M., D. Zarka, J. Coombs, and D.S. Douches. 2019. Comparison of methods to distinguish diploid and tetraploid potato in applied diploid breeding. American Journal of Potato Research 96: 244–254.
Amundson, K., B. Ordonez, M. Santayana, E. Tan, I. Henry, E. Mihovilovich, M. Bonierbale, and L. Comai. 2020. Genomic outcomes of haploid induction crosses in potato (Solanum tuberosum L.). Genetics 214: 369–380.
Arnason, T. 1943. Female sterility in potatoes. Canadian Journal of Research 21: 41–56.
Bethke, P.C., and S.H. Jansky. 2021. Genetic and environmental factors contributing to reproductive success and failure in potato. American Journal of Potato Research 98: 24–41.
Bienz, D.R. 1958. The influence of environmental factors and pollinating techniques on the success of potato pollination in the greenhouse. Am. Potato J. 35: 377–385.
Blomquist, A., and F. Lauer. 1962. A simplified technique for handling and storing potato pollen. Am. Potato J. 39: 340–343.
Caligari, P., W. Powell, K. Liddell, M. De Maine, and G. Swan. 1988. Methods and strategies for detecting Solanum tuberosum dihaploids in interspecific crosses with S. phureja. The Annals of Applied Biology 112: 323–328.
Cipar, M.S., and C. Lawrence. 1972. Scab resistance of haploids from two Solanum tuberosum cultivars. Am. Potato J. 49: 117–119.
Clark, C. 1935. Report of research commitee on potato breeding. Am. Potato J. 12: 91–94.
Clulow, S.a., M.J. Wilkinson, R. Waugh, E. Baird, M.J. DeMaine, and W. Powell. 1991. Cytological and molecular observations on Solanum phureja-induced dihaploid potatoes. Theoretical and Applied Genetics 82: 545–551.
De Maine, M.J. 1977. A simple technique for the short-term storage of pollen of a dihaploid-inducing diploid potato clone. The Journal of Agricultural Science 89: 511–512.
De Maine, M.J. 1988. Berry and seed production using stored pollen of a Solanum phureja clone. Potato res. 31: 385–387.
Dolničar, P., and B. Bohanec. 2000. Ploidy and morphological characteristics of Solanum tuberosum × Solanum phureja hybrids. European Journal of Physiology 439: R9–R11.
Dwivedi, S.L., A.B. Britt, L. Tripathi, S. Sharma, H.D. Upadhyaya, and R. Ortiz. 2015. Haploids: Constraints and opportunities in plant breeding. Biotechnology Advances 33: 812–829.
Edmundson, W. 1939. Comparison of Katahdin potato pollen produced in the field and in the greenhouse. Am. Potato J. 19: 12–15.
Ercolano, M.R., D. Carputo, J. Li, L. Monti, A. Barone, and L. Frusciante. 2004. Assessment of genetic variability of haploids extracted from tetraploid (2n= 4x= 48) Solanum tuberosum. Genome 47: 633–638.
Hanneman, R.E., Jr., and S.J. Peloquin. 1969. Use of Phureja and haploids to enhance the yield of cultivated tetraploid potatoes. Am. Potato J. 46: 436.
Hanneman, R.E., Jr., and R. Ruhde. 1978. Haploid extraction in Solanum tuberosum group Andigena. Am. Potato J. 55: 259–263.
Henderson, M., and E. LeClerg. 1943. Studies of some factors affecting fruit setting in Solanum tuberosum in the field in Louisiana. Journal of Agricultural Research 66: 67–76.
Hermsen, J.G.T., and J. Verdenius. 1973. Selection from Solanum tuberosum group Phureja of genotypes combining high-frequency haploid induction with homozygosity for embryo-spot. Euphytica 22: 244–259.
Hermundstad, S.A., and S.J.J. Peloquin. 1985. Germplasm enhancement with potato haploids. The Journal of Heredity 76: 463–467.
Hougas, R.W., and S.J. Peloquin. 1957. A haploid plant of the potato variety Katahdin. Nature 180: 1209–1210.
Hougas, R.W., and S.J. Peloquin. 1958. The potential of potato haploids in breeding and genetic research. Am. Potato J. 35: 701–707.
Hougas, R., S. Peloquin, and A. Gabert. 1964. Effect of seed parent and pollinator on frequency of haploids in Solaum tuberosum. Crop Science 4: 593–595.
Hougas, R.W., S.J. Peloquin, and R.W. Ross. 1958. Haploids of the common potato. The Journal of Heredity 49: 103–106.
Howard, H.W. 1958. The storage of potato pollen. Am. Potato J. 35: 676–678.
Hutten, R., E. Scholberg, D. Huigen, J.G.T. Hermsen, and E. Jacobsen. 1994. Analysis of dihaploid induction and production ability and seed parent x pollinator interaction in potato. Euphytica 72: 61–64.
Jansky, S.H., A.O. Charkowski, D.S. Douches, G. Gusmini, C. Richael, P.C. Bethke, D.M. Spooner, R.G. Novy, H. De Jong, W.S. De Jong, J.D. Bamberg, A.L. Thompson, B. Bizimungu, D.G. Holm, C.R. Brown, K.G. Haynes, V.R. Sathuvalli, R.E. Veilleux, J.C. Miller Jr., J.M. Bradeen, and J.M. Jiang. 2016. Reinventing potato as a diploid inbred line–based crop. Crop Science 11: 1–11.
Jansky, S.H., Y.S. Chung, and P. Kittipadukal. 2014. M6: A diploid potato inbred line for use in breeding and genetics research. J. Plant Registrations. 8: 195–199.
Jansky, S.H., D.I. Rouse, E.D. Mauritz, J. Clark, and D.I. Rouse. 2003. Disease resistance in potato haploids. Acta Horticulturae 619: 135–144.
Jansky, S., G. Yerk, and S. Peloquin. 1990. The use of potato haploids to put 2x wild species germplasm into a usable form. Plant Breeding 104: 290–294.
King, J.R. 1955. Irish potato pollen storage. Am. Potato J. 32: 460–466.
Kotch, G.P. 1987. The production of haploids and their use in genetic studies in potatoes. PhD. Thesis, University of Wisconsin-Madison, 171 pp.
Kotch, G.P., R. Ortiz, and S.J. Peloquin. 1992. Genetic analysis by use of potato haploid populations. Genome 35: 103–108.
Kotch, G.R., and S. Peloquin. 1987. A new source of haploid germplasm for genetic and breeding research. Am. Potato J. 64: 137–141.
Kramer, L.J., and J. Bamberg. 2019. Comparing methods of ploidy estimation in potato (Solanum) species. American Journal of Potato Research 96: 419–426.
Lindhout, P., D. Meijer, T. Schotte, R.C.B. Hutten, R.G.F. Visser, and H.J. van Eck. 2011. Towards F1 hybrid seed potato breeding. Potato Research 54: 301–312.
Liu, C.A., and D.S. Douches. 1993. Production of haploids of potato (Solanum tuberosum subsp. tuberosum) and their identification with electrophoretic analysis. Euphytica 70: 113–126.
Manrique-Carpintero, N., J. Coombs, G. Pham, F. Laimbeer, T.G. Braz, J. Jiang, R.E. Veilleux, R.C. Buell, and D.S. Douches. 2019. Genome reduction in tetraploid potato reveals genetic load, haplotype variation, and loci associated with agronomic traits. Frontiers in Plant Science 9: 944.
McLean, J.G., and F.J. Stevenson. 1952. Methods of obtaining seed on russet Burbank and similar-flowering varieties of potato. American Potato Journal 29: 206–211.
Montelongo-Escobedo, H., and P.R. Rowe. 1969. Haploid induction in potato: Cytological basis for pollinator effect. Euphytica 18: 116–123.
Ordoñez, B., M. Orrillo and M. Bonierbale. 2017. Manual on potato reproductive and cytological biology. Lima (Peru). International Potato Center (CIP), 65 p.
Ortiz, R., P. Simon, S. Jansky, and D. Stelly. 2009. Ploidy manipulation of the gametophyte, endosperm and sporophyte in nature and for crop improvement: A tribute to Professor Stanley J. Peloquin (1921-2008). Annals of Botany 104: 795–807.
Panahandeh, J. 2010. Dihaploid induction ability of three clones of Solanum phureja (2n = 2x = 24) in interploidy cross with S. tuberosum (2n = 4x = 48). Acta Agron. Hungarica 58 (1): 49–54.
Peloquin, S.J., A.C. Gabert, and R. Ortiz. 1996. Nature of “pollinator” effect in potato (Solanum tuberosum L.) haploid production. Annals of Botany 77: 539–542.
Peloquin, S., and R. Hougas. 1958. Fertility in two haploids of Solanum tuberosum. Science 128: 1340–1342.
Peloquin, S.J., and R.W. Hougas. 1959. Decapitation and genetic markers as related to haploidy in Solanum tuberosum. European Potato Journal 2: 176–183.
Peloquin, S.J., and R.W. Hougas. 1960. Genetic variation among haploids of the common potato. Am. Potato J. 37: 289–297.
Peloquin, S., S.H. Jansky, and G.L. Yerk. 1989. Potato cytogenetics and germplasm utilization. American Journal of Potato Research 66: 629–638.
Rasmussen, J.O., and O.S. Rasmussen. 1995. Characterization of somatic hybrids of potato by use of RAPD markers and isozyme analysis. Physiologia Plantarum 93: 357–364.
Samitsu, Y., and K. Hosaka. 2002. Molecular marker analysis of 24- and 25-chromosome plants obtained from Solanum tuberosum L. subsp. andigena (2n = 4x = 48) pollinated with a Solanum phureja haploid inducer. Genome 45: 577–583.
Sanford, J., and R. Hanneman. 1982. Intermating of potato haploids and spontaneous sexual polyploidization - effects on heterozygosity. American Journal of Potato Research 59: 407–414.
Singsit, C., and R.E. Veilleux. 1991. Chloroplast density in guard cells of leaves of anther-derived potato plants grown in vitro and in vivo. HortScience. 26: 592–594.
Stokstad, E. 2019. The new potato. Science 363: 574–577.
Yeh, B., S.J. Peloquin, and H.R. W. 1964. Meiosis in Solanum tuberosum haploids and haploid-haploid F1 hybrids. Canadian Journal of Genetics and Cytology 6: 393–402.
Zhang, C., P. Wang, D. Tang, Z. Yang, F. Lu, J. Qi, N.R. Tawari, Y. Shang, C. Li, and S. Huang. 2019. The genetic basis of inbreeding depression in potato. Nature Genetics 51: 374–378.
Acknowledgements
Authors wish to thank Andy Hamernik for input, expertise and efforts throughout this work. We also thank Laura Vanderploeg (UW-Madison, biochemistry) for photography and image processing efforts. John Jansky (UW-Madison) assisted with pollinations in 2018 and Asma Alkhaja (UW-Madison) helped process seeds in 2018. Rachel Figueroa (UMN-St. Paul) and Nicole Mihelich (UMN-St. Paul) performed flow cytometry analysis. We appreciate the collection, shoot preparation and pollination efforts of many individuals including: Rachel Figueroa, Katelyn Filbrandt, Caroline Hanson, Jesse Huege, Akpevwe Ikoba, Colin Jones, Alex Knopf, Thomas McGehee, Emily Romdenne, Dakota Schaus, Laura Schulz, Brittany Stokes and Heather Tuttle. Multiple commercial growers assisted in this project including Alsum Farms and Produce, Inc., Friesland, WI; Coloma Farms, Inc., Coloma, WI; Edling Farms, Clear Lake, MN; Ewing Farms Inc., Big Lake, MN; Del Hayes and Sons, Big Lake, MN; Gray Potato Farm, Clear Lake, MN; and Wingard Farms, Elk River, MN. This work was funded by USDA-NIFA award number 2019–51181-30,021 in both states and USDA-NIFA award number 2016–34141-25,707 in Minnesota. This work is dedicated to the loving memory of H. Shirley Busse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busse, J.S., Jansky, S.H., Agha, H.I. et al. A High Throughput Method for Generating Dihaploids from Tetraploid Potato. Am. J. Potato Res. 98, 304–314 (2021). https://doi.org/10.1007/s12230-021-09844-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-021-09844-1