Abstract
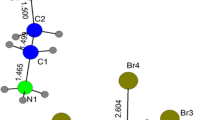
The crystal structures of two polymorphic forms of 1-butyl-3-methylimidazolium nitrate are reported. The form observed at 100 K and 200 K (Ia and Ib) crystallizes in the \(P{\bar{1}}\) space group and contains two independent imidazolium cations and nitrate anions. At 100 K the butyl chain of one cation adopts a TT (trans–trans) conformation and the other cation adopts a G′G′ (gauche–gauche) conformation. At 200 K, the G′G′ chain is disordered with about twelve percent of the GT conformation present. A different polymorph (Form II, also crystallizing in the \(P{\bar{1}}\) space group with two independent ion pairs) is present at 273 K displaying significant disorder in the butyl chains with a mixture of G′T, G′G′, and GT conformers. Raman spectra were collected on samples between 100 and 350 K and show changes in band frequencies and intensities consistent with conversion between different butyl chain conformations. Hydrogen bond interactions are present between cation C–H’s and oxygen atoms of the nitrate ions, with significant lengthening observed for three of the six close contacts (and formation of one new contact) upon conversion to the higher-temperature form. The structural details revealed in this study shed light on the intermolecular forces and the conformational changes that accompany phase changes in 1-butyl-3-methylimidazolium nitrate.
Graphic Abstract
X-ray diffraction and Raman spectroscopy of the ionic liquid 1-butyl- 3-methylimidazolium nitrate at temperatures from 100 to 300 K show evidence of two different polymorphs and significant temperature-dependent conformational changes.










Similar content being viewed by others
Data Availability
Crystallographic data has been deposited with the CCDC and can be obtained free of charge via http://www.ccdc.cam.ac.uk/structures, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cam- bridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
Code Availability
Python code for the conformational analysis of 1-butyl-3-methylimidazolium ions using the CSD Python API is freely available at https://gitlab.com/djohnston66/bmim-analysis.
References
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99(8):2071–2084. https://doi.org/10.1021/cr980032t
Hallett JP, Welton T (2011) Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev 111(5):3508–3576. https://doi.org/10.1021/cr1003248
Fedorov MV, Kornyshev AA (2014) Ionic liquids at electrified interfaces. Chem Rev 114(5):2978–3036. https://doi.org/10.1021/cr400374x
Hayes R, Warr GG, Atkin R (2015) Structure and nanostructure in ionic liquids. Chem Rev 115(13):6357–6426. https://doi.org/10.1021/cr500411q
Dean PM, Pringle JM, MacFarlane DR (2010) Structural analysis of low melting organic salts: perspectives on ionic liquids. Phys Chem Chem Phys 12(32):9144–9153. https://doi.org/10.1039/C003519J
Beichel W, Preiss UP, Benkmil B, Steinfeld G, Eiden P, Kraft A, Krossing I (2013) Temperature dependent crystal structure analyses and ion volume determinations of organic salts. Z Anorg Allg Chem 639(12–13):2153–2161. https://doi.org/10.1002/zaac.201300246
Choudhury AR, Winterton N, Steiner A, Cooper AI, Johnson KA (2005) In situ crystallization of low-melting ionic liquids. J Am Chem Soc 127(48):16792–16793. https://doi.org/10.1021/ja055956u
Abe H, Kishimura H, Takekiyo T, Hanasaki T, Yoshimura Y, Hamaya N (2020) Low-temperature and high-pressure phase changes of room-temperature ionic liquids. J Mol Liq 300:112340. https://doi.org/10.1016/j.molliq.2019.112340
Swatloski RP, Holbrey JD, Rogers RD (2003) Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem 5(4):361–363. https://doi.org/10.1039/B304400A
Bouvet S, Pégot B, Marrot J, Magnier E (2014) Solvent free nucleophilic introduction of fluorine with [bmim][F]. Tetrahedron Lett 55(4):826–829. https://doi.org/10.1016/j.tetlet.2013.12.020
Holbrey JD, Reichert WM, Nieuwenhuyzen M, Johnson S, Seddon KR, Rogers RD (2003) Crystal polymorphism in 1-butyl-3-methylimidazolium halides: supporting ionic liquid formation by inhibition of crystallization. Chem Commun 14:1636. https://doi.org/10.1039/B304543A
Saha S, Hayashi S, Kobayashi A, Hamaguchi H-O (2003) Crystal structure of 1-Butyl-3-methylimidazolium Chloride. A clue to the elucidation of the ionic liquid structure. Chem Lett 32(8):740–741. https://doi.org/10.1246/cl.2003.740
Kärkkäinen J, Asikkala J, Laitinen RS, Lajunen MK (2004) Effect of temperature on the purity of product in the preparation of 1-butyl-3-methylimidazolium-based ionic liquids. Z Naturforsch B 59(7):763–770. https://doi.org/10.1515/znb-2004-0704
Pulham C, Pringle J, Parkin A, Parsons S, Messenger D (2005) CSD Communication. https://doi.org/10.5517/CC992J5
Ozawa R, Hayashi S, Saha S, Kobayashi A, Hamaguchi H-O (2003) Rotational isomerism and structure of the 1-butyl-3-methylimidazolium cation in the ionic liquid state. Chem Lett 32(10):948–949. https://doi.org/10.1246/cl.2003.948
Vygodskii YS, Mel'nik OA, Lozinskaya EI, Shaplov AS, Malyshkina IA, Gavrilova ND, Lyssenko KA, Antipin MY, Golovanov DG, Korlyukov AA, Ignat’ev N, Welz-Biermann U (2007) The influence of ionic liquid’s nature on free radical polymerization of vinyl monomers and ionic conductivity of the obtained polymeric materials. Polym Adv Technol 18(1):50–63. https://doi.org/10.1002/pat.795
Dibrov SM, Kochi JK (2006) Crystallographic view of fluidic structures for room-temperature ionic liquids: 1-butyl-3-methylimidazolium hexafluorophosphate. Acta Crystallogr C 62(1):o19–o21. https://doi.org/10.1107/S0108270105037200
Saouane S, Norman SE, Hardacre C, Fabbiani FPA (2013) Pinning down the solid-state polymorphism of the ionic liquid [bmim][PF6]. Chem Sci 4(3):1270. https://doi.org/10.1039/C2SC21959J
Dupont J, Suarez PAZ, De Souza RF, Burrow RA, Kintzinger J-P (2000) CH-π interactions in 1-n-butyl-3-methylimidazolium tetraphenylborate molten salt: solid and solution structures. Chem Eur J 6(13):2377–2381. https://doi.org/10.1002/1521-3765(20000703)6:13%3C2377::AID-CHEM2377%3E3.0.CO;2-L
Stenzel O, Raubenheimer HG, Esterhuysen C (2002) Biphasic hydroformylation in new molten salts–analogies and differences to organic solvents. J Chem Soc Dalton Trans 6:1132. https://doi.org/10.1039/B107720A
van den Broeke J, Stam M, Lutz M, Kooijman H, Spek AL, Deelman B-J, van Koten G (2003) Designing ionic liquids: 1-butyl-3-methylimidazolium cations with substituted tetraphenylborate counterions. Eur J Inorg Chem 2003(15):2798–2811. https://doi.org/10.1002/ejic.200300057
Finden J, Beck G, Lantz A, Walsh R, Zaworotko MJ, Singer RD (2003) Preparation and characterization of 1-butyl-3-methylimidazolium tetrakis(3,5-bis(trifluoromethyl) phenyl)borate, [bmim]BARF. J Chem Crystallogr 33(4):287–295. https://doi.org/10.1023/A:1023885212233
Zhang B, Köberl M, Pöthig A, Cokoja M, Herrmann WA, Kühn FE (2012) Synthesis and characterization of imidazolium salts with the weakly coordinating [B(C6F5)4]- anion. Z Naturforsch B 67(10):1030–1036. https://doi.org/10.5560/znb.2012-0180
Paulechka YU, Kabo GJ, Blokhin AV, Shaplov AS, Lozinskaya EI, Golovanov DG, Lyssenko KA, Korlyukov AA, Vygodskii YS (2009) IR and X-ray Study of Polymorphism in 1-Alkyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)imides. J Phys Chem B 113(28):9538–9546. https://doi.org/10.1021/jp903702c
Santos CS, Rivera-Rubero S, Dibrov S, Baldelli S (2007) Ions at the surface of a room-temperature ionic liquid. J Phys Chem C 111(21):7682–7691. https://doi.org/10.1021/jp0652751
Kuzmina O, Hassan NH, White AJ, Welton T (2017) CSD Communication. https://doi.org/10.5517/ccdc.csd.cc1p2p1h
Finger LH, Sundermeyer J (2016) Halide-free synthesis of hydrochalcogenide ionic liquids of the type [cation][HE] (E=S, Se, Te). Chem Eur J 22(12):4218–4230. https://doi.org/10.1002/chem.201504577
Hayashi S, Ozawa R, Hamaguchi H-O (2003) Raman spectra, crystal polymorphism, and structure of a prototype ionic-liquid [bmim]Cl. Chem Lett 32(6):498–499. https://doi.org/10.1246/cl.2003.498
Endo T, Nishikawa K (2013) Thermal phase behavior of 1-butyl-3-methylimidazolium hexafluorophosphate: simultaneous measurements of the melting of two polymorphic crystals by Raman spectroscopy and calorimetry. Chem Phys Lett 584:79–82. https://doi.org/10.1016/j.cplett.2013.08.052
Cao W, Wang Y, Saielli G (2018) Metastable state during melting and solid-solid phase transition of [CnMim][NO3] (n = 4–12) ionic liquids by molecular dynamics simulation. J Phys Chem B 122(1):229–239. https://doi.org/10.1021/acs.jpcb.7b09073
Strechan AA, Kabo AG, Paulechka YU, Blokhin AV, Kabo GJ, Shaplov AS, Lozinskaya EI (2008) Thermochemical properties of 1-butyl-3-methylimidazolium nitrate. Thermochim Acta 474(1):25–31. https://doi.org/10.1016/j.tca.2008.05.002
Endo T, Hoshino S, Shimizu Y, Fujii K, Nishikawa K (2016) Comprehensive conformational and rotational analyses of the butyl group in cyclic cations: DFT calculations for imidazolium, pyridinium, pyrrolidinium, and piperidinium. J Phys Chem B 120(39):10336–10349. https://doi.org/10.1021/acs.jpcb.6b07166
Abe H, Takekiyo T, Yoshimura Y, Saihara K, Shimizu A (2016) Anomalous freezing of nano-confined water in room-temperature ionic liquid 1-butyl-3-methylimidazolium nitrate. ChemPhysChem 17(8):1136–1142. https://doi.org/10.1002/cphc.201501199
Talaty ER, Raja S, Storhaug VJ, Dölle A, Carper WR (2004) Raman and infrared spectra and ab initio calculations of C2−4 MIM imidazolium hexafluorophosphate ionic liquids. J Phys Chem B 108(35):13177–13184. https://doi.org/10.1021/jp040199s
Berg RW, Deetlefs M, Seddon KR, Shim I, Thompson JM (2005) Raman and ab initio studies of simple and binary 1-alkyl-3-methylimidazolium ionic liquids. J Phys Chem B 109(40):19018–19025. https://doi.org/10.1021/jp050691r
Heimer NE, Del Sesto RE, Meng Z, Wilkes JS, Carper WR (2006) Vibrational spectra of imidazolium tetrafluoroborate ionic liquids. J Mol Liq 124(1–3):84–95. https://doi.org/10.1016/j.molliq.2005.08.004
Holomb R, Martinelli A, Albinsson I, Lassègues JC, Johansson P, Jacobsson P (2008) Ionic liquid structure: the conformational isomerism in 1-butyl-3-methyl-imidazolium tetrafluoroborate ([bmim][BF4]). J Raman Spectrosc 39(7):793–805. https://doi.org/10.1002/jrs.1912
Hamaguchi H-O, Ozawa R (2005) Structure of ionic liquids and ionic liquid compounds: are ionic liquids genuine liquids in the conventional sense? Adv Chem Phys 131:85–104
Berg RW (2007) Raman spectroscopy and Ab-initio model calculations on ionic liquids. Monatsh Chem 138(11):1045–1075. https://doi.org/10.1007/s00706-007-0760-9
Saha S, Hiroi T, Iwata K, Hamaguchi H-O (2015) Raman spectroscopy and the heterogeneous liquid structure in ionic liquids. In: Plechkova NV, Seddon KR (eds) Ionic liquids completely uncoiled: critical expert overviews. Wiley, Hoboken, pp 165–187
Paschoal VH, Faria LFO, Ribeiro MCC (2017) Vibrational spectroscopy of ionic liquids. Chem Rev 117(10):7053–7112. https://doi.org/10.1021/acs.chemrev.6b00461
Kausteklis J, Talaikis M, Aleksa V, Balevičius V (2018) Raman spectroscopy study of water confinement in ionic liquid 1-butyl-3-methylimidzolium nitrate. J Mol Liq 271:747–755. https://doi.org/10.1016/j.molliq.2018.09.060
Gruzdev MS, Ramenskaya LM, Chervonova UV, Kumeev RS (2009) Preparation of 1-butyl-3-methylimidazolium salts and study of their phase behavior and intramolecular intractions. Russ J Gen Chem 79(8):1720–1727. https://doi.org/10.1134/S1070363209080246
Solar M, Trapp N (2018) μCHILL: a lightweight, modular system for handling crystalline samples at low temperatures under inert conditions. J Appl Crystallogr 51(2):541–548. https://doi.org/10.1107/S1600576718003291
Bruker (2019) APEX3, SAINT and SADABS. Bruker AXS Inc., Madison, WI
Sheldrick GM (2015) SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr A 71(1):3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr C 71(1):3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42(2):339–341. https://doi.org/10.1107/S0021889808042726
Palmer D (2020) CrystalMaker®: a crystal and molecular structures program for Mac and Windows. CrystalMaker Software Ltd, Oxford
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge structural database. Acta Crystallogr B 72(2):171–179. https://doi.org/10.1107/S2052520616003954
Daylight Chemical Information Systems (2019) SMARTS - A Language for Describing Molecular Patterns. https://www.daylight.com/dayhtml/doc/theory/theory.smarts.html
Spartan ’18 (2018) Wavefunction, Inc., Irvine, CA
Wilkes JS, Zaworotko MJ (1992) Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J Chem Soc Chem Commun. https://doi.org/10.1039/C39920000965
Tsuzuki S, Tokuda H, Mikami M (2007) Theoretical analysis of the hydrogen bond of imidazolium C2-H with anions. Phys Chem Chem Phys 9(34):4780. https://doi.org/10.1039/B707419K
Fumino K, Wulf A, Ludwig R (2009) The potential role of hydrogen bonding in aprotic and protic ionic liquids. Phys Chem Chem Phys 11(39):8790. https://doi.org/10.1039/B905634C
Dong K, Zhang S, Wang J (2016) Understanding the hydrogen bonds in ionic liquids and their roles in properties and reactions. Chem Commun 52(41):6744–6764. https://doi.org/10.1039/C5CC10120D
Spek AL (2015) PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr C 71(1):9–18. https://doi.org/10.1107/S2053229614024929
Nakakoshi M, Shiro M, Fujimoto T, Machinami T, Seki H, Tashiro M, Nishikawa K (2006) Crystal structure of 1-Butyl-3-methylimidazolium iodide. Chem Lett 35:1400–1401. https://doi.org/10.1246/cl.2006.1400
Funding
This work was supported in part by the National Science Foundation through Grant No. CHE-0942850.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest or competing interests.
Rights and permissions
About this article
Cite this article
Johnston, D.H., Gasbarre, M. & Norris, C.E. Structural and Conformational Analysis of 1-Butyl-3-methylimidazolium Nitrate. J Chem Crystallogr 52, 140–151 (2022). https://doi.org/10.1007/s10870-021-00899-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00899-w