Abstract
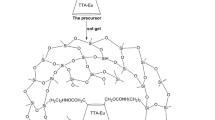
In the search for appropriate host matrices for highly luminescent molecular guest species, an organo-silica monolithic xerogel prepared by the hydrolysis and co-condensation reactions of 3-glycidoxypropyltrimethoxysilane (GPTS) and tetraethylorthosilicate (TEOS) is proposed. Such alkoxides allowed the development of hybrid silica monoliths with a cross-linked three-dimensional structure, in which the silica domains are strictly interconnected with the polymeric network. Highly luminescent monoliths were prepared from the immobilization of a blue-green emissive Ir(III) phosphor in the host matrix, with improved luminescent properties attributed to the greater rigidity of the medium and less diffusion of oxygen within the matrix. The structure of the hybrid material was elucidated by high-resolution solid-state NMR, confirming that the final structure of the developed silica matrix is very similar for both Ir(III)-doped and undoped xerogels. Altogether, the experimental strategy used in this work stands as an advance in the design of photo-functional materials with substantial potential for optical and photonic applications.

Highlights
-
Highly luminescent GPTS:TEOS-derived organosilicate monolithic xerogel is presented.
-
Structural description of the matrix using high-resolution solid-state NMR techniques.
-
Improved luminescent properties due to the rigid media and lower diffusion of O2.
-
Versatile hybrid host system promising for optical and luminescent applications.












Similar content being viewed by others
References
Pagliaro M (2009) Silica-Based Materials for Advanced Chemical Applications. The Royal Society of Chemistry, Cambridge CB4 0WF, UK, p 192. ISBN 978-1847558985.
Ciriminna R, Fidalgo A, Pandarus V et al. (2013) The sol-gel route to advanced silica-based materials and recent applications. Chem Rev 113:6592–6620. https://doi.org/10.1021/cr300399c
Schmidt H (2006) Considerations about the sol-gel process: from the classical sol-gel route to advanced chemical nanotechnologies. J Sol-Gel Sci Technol 40:115–130. https://doi.org/10.1007/s10971-006-9322-6
Sanchez C, Belleville P, Popall M, Nicole L (2011) Hybrid materials themed issue Cluster-based inorganic-organic hybrid materialsw. Chem Soc Rev 40:696–753. https://doi.org/10.1039/c0cs00136h
Wencel D, Barczak M, Borowski P, McDonagh C (2012) The development and characterisation of novel hybrid sol-gel-derived films for optical pH sensing. J Mater Chem 22:11720–11729. https://doi.org/10.1039/c2jm31240a
Schottner G (2001) Hybrid sol-gel-derived polymers: applications of multifunctional materials. Chem Mater 13:3422–3435. https://doi.org/10.1021/cm011060m
Barczak M, Mcdonagh C, Wencel D (2016) Micro- and nanostructured sol-gel-based materials for optical chemical sensing (2005 – 2015). Microchim Acta 183:2085–2109. https://doi.org/10.1007/s00604-016-1863-y
Zanoni KPS, Ravaro LP, De Camargo ASS (2018) Host-guest luminescent materials based on highly emissive species loaded into versatile sol-gel hosts. Dalt Trans 47:12813–12826
Lukowiak A, Strek W (2009) Sensing abilities of materials prepared by sol-gel technology. J Sol-Gel Sci Technol 50:201–215. https://doi.org/10.1007/s10971-009-1952-z
Awano CM, De Vicente FS, Donatti DA, Vollet DR (2012) Structure and growth kinetics of 3-glycidoxypropyltrimethoxysilane-derived organic/silica hybrids at different temperatures. J Phys Chem C 116:24274–24280. https://doi.org/10.1021/jp305222z
Vollet DR, Barreiro LA, Awano CM et al. (2017) Rod-like particles growing in sol – gel processing of 1: 1 molar mixtures of 3-glycidoxypropyltrimeth- oxysilane and tetraethoxysilane research papers. J Appl Crystallogr 50:489–497. https://doi.org/10.1107/S1600576717002357
Alencar LDS, Pilla V, Andrade AA et al. (2014) High fluorescence quantum efficiency of CdSe/ZnS quantum dots embedded in GPTS/TEOS-derived organic/silica hybrid colloids. Chem Phys Lett 599:63–67. https://doi.org/10.1016/j.cplett.2014.03.016
Ferreira PHD, Otuka AJG, Barbano EC et al. (2015) Femtosecond laser fabrication of waveguides in Rhodamine B-doped GPTS / TEOS-derived organic / silica monolithic xerogel. Opt Mater (Amst) 47:310–314. https://doi.org/10.1016/j.optmat.2015.05.047
Abegão LMG, Manoel DS, Otuka AJG et al. (2017) Random laser emission from a Rhodamine B-doped GPTS/TEOS-derived organic/silica monolithic xerogel. Laser Phys Lett 14:0–6. https://doi.org/10.1088/1612-202X/aa699b
de Vicente FS, Freddi P, Otuka AJG et al. (2018) Photoluminescence tuning and energy transfer process from Tb3+ to Eu3+ in GPTMS/TEOS–derived organic/silica hybrid films. J Lumin 197:370–375. https://doi.org/10.1016/j.jlumin.2017.12.048
Evans RC, Douglas P, Winscom CJ (2006) Coordination complexes exhibiting room-temperature phosphorescence: evaluation of their suitability as triplet emitters in organic light emitting diodes. Coord Chem Rev 250:2093–2126. https://doi.org/10.1016/j.ccr.2006.02.007
Kalyanasundaram K, Gra ̈tzel M (1998) Applications of functionalized transition metal complexes in photonic and optoelectronic devices. Commun Chem Rev 177:347–414. https://doi.org/10.1016/S0010-8545(98)00189-1
You Y, Park SY (2008) Phosphorescent iridium(iii) complexes: toward high phosphorescence quantum efficiency through ligand control. J Chem Soc Dalt Trans 9226:1267–1282. https://doi.org/10.1039/b812281d
Zanoni KPS, Ito A, Grüner M et al. (2018) Photophysical dynamics of the efficient emission and photosensitization of [Ir(: Pqi)2(NN)]+ complexes. Dalt Trans 47:1179–1188. https://doi.org/10.1039/c7dt03930a
Zanoni KPS, Coppo RL, Amaral RC, Murakami Iha NY (2015) Ir(III) complexes designed for light-emitting devices: beyond the luminescence color array. Dalt Trans 44:14559–14573. https://doi.org/10.1039/c5dt01644d
Smith ARG, Burn PL, Powell BJ (2011) Spin-orbit coupling in phosphorescent iridium(III) complexes. ChemPhysChem 12:2429–2438. https://doi.org/10.1002/cphc.201100397
Flamigni L, Barbieri A, Sabatini C et al. (2007) Photochemistry and Photophysics of Coordination Compounds: Iridium. In: Photochemistry and Photophysics of Coordination Compounds. Topics in Current Chemistry, 281. Springer, Berlin, Heidelberg. p 143–203. https://doi.org/10.1007/128_2007_131
Yersin H, Rausch AF, R Czerwieniec TH and TF (2011) Charge-Transfer Excited States in Phosphorescent Organo-Transition Metal Compounds: a Difficult Case for Time Dependent Density Functional Theory? Coord Chem Rev 255:2622–2652. https://doi.org/10.1039/C5RA12962A
Zanoni KPS, Vilela RRC, Silva IDA et al. (2019) Photophysical Properties of Ir(III) Complexes Immobilized in MCM-41 via Templated Synthesis. Inorg Chem 58:4962–4971. https://doi.org/10.1021/acs.inorgchem.8b03633
Grüner MC, Zanoni KPS, Borgognoni CF et al. (2018) Reaching Biocompatibility with Nanoclays: eliminating the Cytotoxicity of Ir(III) Complexes. ACS Appl Mater Interfac 10:26830–26834. https://doi.org/10.1021/acsami.8b10842
Zanoni KPS, Kariyazaki BK, Ito A et al. (2014) Blue-green iridium(III) emitter and comprehensive photophysical elucidation of heteroleptic cyclometalated iridium(III) complexes. Inorg Chem 53:4089–4099. https://doi.org/10.1021/ic500070s
Jaeger C, Hemmann F (2014) EASY: a simple tool for simultaneously removing background, deadtime and acoustic ringing in quantitative NMR spectroscopy - Part I: Basic principle and applications. Solid State Nucl Magn Reson 57–58:22–28. https://doi.org/10.1016/j.ssnmr.2013.11.002
Bennett AE, Rienstra CM, Auger M et al. (1995) Heteronuclear decoupling in rotating solids. J Chem Phys 103:6951–6958. https://doi.org/10.1063/1.470372
Kimura H, Nakamura K, Eguchi A et al. (1998) Structural study of α-amino-acid crystals by 1H CRAMPS NMR spectroscopy. J Mol Struct 447:247–255. https://doi.org/10.1016/S0022-2860(98)00329-9
Potrzebowski MJ, Tekely P, Dusausoy Y (1998) Comment to 13C-NMR studies of α and γ polymorphs of glycine. Solid State Nucl Magn Reson 11:253–257. https://doi.org/10.1016/S0926-2040(98)00028-9
Baccile N, Laurent G, Bonhomme C et al. (2007) Solid-state NMR characterization of the surfactant-silica interface in templated silicas: acidic versus basic conditions. Chem Mater 19:1343–1354. https://doi.org/10.1021/cm062545j
Christiansen SC, Hedin N, Epping JD et al. (2006) Sensitivity considerations in polarization transfer and filtering using dipole-dipole couplings: Implications for biomineral systems. Solid State Nucl Magn Reson 29:170–182. https://doi.org/10.1016/j.ssnmr.2005.10.010
Wiench JW, Bronnimann CE, Lin VSY, Pruski M (2007) Chemical shift correlation NMR spectroscopy with indirect detection in fast rotating solids: Studies of organically functionalized mesoporous silicas. J Am Chem Soc 129:12076–12077. https://doi.org/10.1021/ja074746+
Massiot D, Dion P, Alcover JF, Bergaya F (1995) 27Al and 29Si MAS NMR Study of Kaolinite Thermal Decomposition by Controlled Rate Thermal Analysis. J Am Ceram Soc 78:2940–2944
Launer PJ (2013) Infrared analysis of organosilicon compounds: spectra-structure correlations. In: Arkles B, Larson GL (eds) Silicon Compounds: Silanes & Silicones, 3rd edn. Gelest Inc., Morrisville PA, p 175–178
Fontinha IR, Salta MM, Zheludkevich ML, Ferreira MGS (2013) EIS Study of Amine Cured Epoxy-silica-zirconia Sol-gel Coatings for Corrosion Protection of the Aluminium Alloy EN AW 6063. Port Electrochim Acta 31:307–319. https://doi.org/10.4152/pea.201306307
Chan Z, Ai’mei L, Xiao Z et al. (2007) Microstructures and properties of ORMOSIL comprising methyl, vinyl, and γ-glycidoxypropyl-substitued silica. Opt Mater (Amst) 29:1543–1547. https://doi.org/10.1016/j.optmat.2006.08.014
Aujara KM, Chieng BW, Ibrahim NA et al. (2019) Gamma-irradiation induced functionalization of graphene oxide with organosilanes. Int J Mol Sci 20. https://doi.org/10.3390/ijms20081910
Shukla DK, Kasisomayajula SV, Parameswaran V (2008) Epoxy composites using functionalized alumina platelets as reinforcements. Compos Sci Technol 68:3055–3063. https://doi.org/10.1016/j.compscitech.2008.06.025
Šapić IM, Bistričić L, Volovšek V et al. (2009) DFT study of molecular structure and vibrations of 3-glycidoxypropyltrimethoxysilane. Spectrochim Acta - Part A Mol Biomol Spectrosc 72:833–840. https://doi.org/10.1016/j.saa.2008.11.032
Šapic̈ IM, Bistričic̈ L, Volovšek V, Dananic̈ V (2014) Vibrational analysis of 3-glycidoxypropyltrimethoxysilane polymer. Macromol Symp 339:122–129. https://doi.org/10.1002/masy.201300145
Posset U, Lankers M, Kiefer W et al. (1993) Polarized raman spectra from some sol-gel precursors and micro-raman study of one selected copolymer. Appl Spectrosc 47:1600–1603. https://doi.org/10.1366/0003702934334787
Riegel B, Blittersdorf S, Kiefer W et al. (1998) Kinetic investigations of hydrolysis and condensation of the glycidoxypropyltrimethoxysilane/aminopropyltriethoxy-silane system by means of FT-Raman spectroscopy I. J Non Cryst Solids 226:76–84. https://doi.org/10.1016/S0022-3093(97)00487-0
Engelhardt G, Michel D (1987) High Resolution Solid State NMR of Silicates and Zeolites. John Wiley & Sons, Ltd, New York, USA
Templin M, Wiesner U, Spiesss HW (1997) Multinuclear solid-state-NMR studies of hybrid organic-inorganic materials. Adv Mater 9:814–817. https://doi.org/10.1002/adma.19970091011
de Buyl F, Kretschmer A (2008) Understanding hydrolysis and condensation kinetics of γ-glycidoxypropyltrimethoxysilane. J Adhes 84:125–142. https://doi.org/10.1080/00218460801952809
Innocenzi P, Figus C, Kidchob T et al. (2009) Sol-gel reactions of 3-glycidoxypropyltrimethoxysilane in a highly basic aqueous solution. Dalt Trans 9146–9152. https://doi.org/10.1039/b905830c
Babij NR, McCusker EO, Whiteker GT et al. (2016) NMR Chemical Shifts of Trace Impurities: industrially Preferred Solvents Used in Process and Green Chemistry. Org Process Res Dev 20:661–667. https://doi.org/10.1021/acs.oprd.5b00417
Gottlieb HE, Kotlyar V, Nudelman A (1997) NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J Org Chem 62:7512–7515. https://doi.org/10.1021/jo971176v
Buntkowsky G, Breitzke H, Adamczyk A et al. (2007) Structural and dynamical properties of guest molecules confined in mesoporous silica materials revealed by NMR. Phys Chem Chem Phys 9:4843. https://doi.org/10.1039/b707322d
Marin V, Holder E, Hoogenboom R et al. (2006) Light-emitting iridium(III) and ruthenium(II) polypyridyl complexes containing quadruple hydrogen-bonding moieties. https://doi.org/10.1039/b513957k
Zanoni KPS, Murakami Iha NY (2016) Sky-blue OLED through PVK:[Ir(Fppy)2(Mepic)] active layer. Synth Met 222:393–396. https://doi.org/10.1016/j.synthmet.2016.11.006
Moore SA, Davies DL, Karim MM et al. (2013) Photophysical behaviour of cyclometalated iridium(iii) complexes with phosphino(terthiophene) ligands. Dalt Trans 42:12354–12363. https://doi.org/10.1039/c3dt51320c
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) Finance Code 001, and supported by the Center for Research, Technology and Education in Vitreous Materials (CeRTEV), Project 2013/07793-6, funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). MO Jr acknowledges the National Council for Scientific and Technological (CNPq, grant n° 311069/2020-7). FSdeV acknowledges FAPESP (grant n° 2011/18149-5) and CNPq (grants n° 427220/2018-1 and 444810/2014-5). Special thanks are given to Professor Paulo Sérgio Pizani for assistance in Raman Spectroscopy and Me. Germán Darío Gómez Higuita for his assistance in Fourier Transformed Infrared Spectroscopy (FTIR).
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) Finance Code 001, and supported by the Center for Research, Technology and Education in Vitreous Materials (CeRTEV), Project 2013/07793-6, funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vilela, R.R.C., Zanoni, K.P.S., de Oliveira, M. et al. Structural and photophysical characterization of highly luminescent organosilicate xerogel doped with Ir(III) complex. J Sol-Gel Sci Technol 102, 236–248 (2022). https://doi.org/10.1007/s10971-021-05593-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05593-z