Abstract
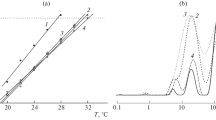
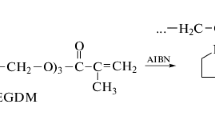
The complexes of metformin (MET) and the copolymer of N-vinylpyrrolidone with triethylene glycol dimethacrylate with absolute molecular weight ca. 26 kDa and hydrodynamic radius of macromolecules about 4 nm have been prepared and studied in water buffer solution. Their sizes in neutral phosphate buffer solution depended on MET (10–40 wt%) per the copolymer content, temperature and the copolymer concentrations. Quantum chemical simulation has shown the most energetically favorable and stable structure is one with the coordination of neighboring NH2 groups of MET via oxygen carbonyl of the VP fragment. The effective binding constant of MET with the copolymer estimated from the absorption spectroscopy was found to be ~ 1.8 × 103 M−1. The MET-copolymer compositions were analyzed by IR spectroscopy, thermogravimetry, differential scanning calorimetry, and X-ray diffraction. The copolymer complex with 20 wt% of MET affected blood glucose level in streptozotocin-induced diabetic mice like MET and decreased aldose reductase activity in contrast with the drug and MET-PVP complex.












Similar content being viewed by others
References
Mandal AK (2020) Dendrimers in targeted drug delivery applications: a review of diseases and cancer. Int J Polym Mater Polym Biomater 7:1–11. https://doi.org/10.1080/00914037.2020.1713780
Kalomiraki M, Thermos K, Chaniotakis NA (2016) Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int J Nanomed 11:1–12. https://doi.org/10.2147/IJN.S93069
Singh J, Jain K, Mehra NK, Jain NK (2016) Dendrimers in anticancer drug delivery: mechanism of interaction of drug and dendrimers. Artific Cells Nanomed Biotechnol 44:1626–1634. https://doi.org/10.3109/21691401.2015.1129625
Lombardo D (2014) Modeling dendrimers charge interaction in solution: Relevance in biosystems. Biochem Res Int 837651:1–10. https://doi.org/10.1155/2014/837651
Kannan RM, Nance E, Kannan S, Tomalia DA (2014) Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J Intern Med 276:579–617. https://doi.org/10.1111/joim.12280
Agrawal A, Kulkarni S (2015) Dendrimers: A new generation carrier. Int J Res Dev Pharm Life Sci 4:1700–1712. https://ijrdpl.com/index.php/ijrdpl/article/view/433
Gao C, Yan D (2004) Hyperbranched polymers: from synthesis to applications. Progr Polym Sci 29:183–275. https://doi.org/10.1016/j.progpolymsci.2003.12.002
Niels M, Smeets B (2013) Amphiphilic hyperbranched polymers from the copolymerization of a vinyl and divinyl monomer: The potential of catalytic chain transfer polymerization. Eur Polym J 49:2528–2544. https://doi.org/10.1016/j.eurpolymj.2013.05.006
Paleos CM, Tsiourvas D, Sideratou Z, Tziveleka L-A (2010) Drug delivery using multifunctional dendrimers and hyperbranched polymers. Exp Opin Drug Deliv 7:1387–1398. https://doi.org/10.1517/17425247.2010.534981
Zeng X, Zhang Y, Wu Z, Lundberg P, Malkoch M, Nyström AM (2011) Hyperbranched copolymer micelles as delivery vehicles of doxorubicin in breast cancer cells. J Polym Sci A: Polym Chem 50:280–288. https://doi.org/10.1002/pola.25027
Zhou Y, Yan D (2009) Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: progress, characteristics and perspectives. Chem Commun 10:1172–1188. https://doi.org/10.1039/B814560C
Zhou Y, Huang W, Liu J, Zhu X, Yan D (2010) Self-assembly of hyperbranched polymers and its biomedical applications. Adv Mater 22:4567–4590. https://doi.org/10.1002/adma.201000369
Voit BI, Lederer A (2009) Hyperbranched and highly branched polymer architectures – synthetic strategies and major characterization aspects. Chem Rev 109:5924–5973. https://doi.org/10.1021/cr900068q
Tonhauser C, Schüll C, Dingels C, Frey H (2012) Branched acid-degradable, biocompatible polyether copolymers via anionic ring−opening polymerization using an epoxide inimer. ACS Macro Lett 1:1094–1097. https://doi.org/10.1021/mz300265z
Vogt AP, Sumerlin BS (2008) Tuning the temperature response of branched poly(N-isopropylacrylamide) prepared by RAFT polymerization. Macromolecules 41:7368–7373. https://doi.org/10.1021/ma801256k
Wang Z, He J, Tao Y, Yang L, Jiang H, Yang Y (2003) Controlled chain branching by RAFT−based radical polymerization. Macromolecules 36:7446–7452. https://doi.org/10.1021/ma025673b
Malkoch M, Schleicher K, Drockenmuller E, Hawker CJ, Russell TP, Wu P, Malkoch M, Schleicher K, Drockenmuller E, Hawker CJ, Russell TP, Wu P, Fokin VV (2005) Structurally diverse dendritic libraries: a highly efficient functionalization approach using click chemistry. Macromolecules 38:3663–3678. https://doi.org/10.1021/ma047657f
Smeets NMB, Freeman MW, McKenna TFL (2011) Polymer architecture control in emulsion polymerization via catalytic chain transfer polymerization. Macromolecules 44:6701–6710. https://doi.org/10.1021/ma201393b
O’Brien N, McKee A, Sherrington DC, Slark AT, Titterton A (2000) Facile, versatile and cost effective route to branched vinyl polymers. Polymer 41:6027–6031. https://doi.org/10.1016/S0032-3861(00)00016-1
Luzon M, Boyer C, Peinado C, Corrales T, Whittaker M, Tao L, Davis TP (2010) Water soluble, thermoresponsive, hyperbranched copolymers based on PEG methacrylates: Synthesis, characterization, and LCST behavior. J Polym Sci A: Polym Chem 48:2783–2792. https://doi.org/10.1002/pola.24027
Chambon P, Chen L, Furzeland S, Atkins D, Weaver JVM, Adams DJ (2011) Poly(N-isopropylacrylamide) branched polymer nanoparticles. Polym Chem 2:941–949. https://doi.org/10.1039/C0PY00369G
Besenius P, Slavin S, Vilela F, Sherrington DC (2008) Synthesis and characterization of water soluble densely branched glycopolymers. React Funct Polym 68:1524–1533. https://doi.org/10.1016/j.reactfunctpolym.2008.08.004
Kurmaz SV, Pyryaev AN (2010) Synthesis of N-vinyl-2-pyrrolidone-based branched copolymers via crosslinking free-radical copolymerization in the presence of a chain-transfer agent. Polymer Sci B 52:1–8. https://doi.org/10.1134/S156009041001001X
Kurmaz SV, Obraztsova NA, Balakina AA, Terent’ev AA (2016) Preparation of the amphiphilic copolymer of N-vinylpyrrolidone with triethylene glycol dimethacrylate nanoparticles and the study of their properties in vitro. Russ Chem Bull 65:2097–2102. https://doi.org/10.1007/s11172-016-1558-x
Kurmaz SV, Obraztsova NA, Perepelitsina EO, Shilov GV, Anokhin DV, Pechnikova EV (2015) New hybrid macromolecular structures of C60 fullerene–amphiphilic copolymers of N-vinylpyrrolidone and triethylene glycol dimethacrylate. Materials Today Communications 4:130–140. https://doi.org/10.1016/j.mtcomm.2015.05.004
Kurmaz SV, Gak VYu, Kurmaz VA, Konev DV (2018) Preparation and properties of hybrid nanostructures of zinc tetraphenylporphyrinate and an amphiphilic copolymer of N-Vinylpyrrolidone in a neutral aqueous buffer solution. Russ J Phys Chem A 92:329–333. https://doi.org/10.1134/S0036024418020152
Kurmaz SV, Rudneva TN, Sanina NA (2018) New nitric oxide-carrier systems based on an amphiphilic copolymer of N-vinylpyrrolidone with triethylene glycol dimethacrylate. Mendeleev Commun 28:73–75. https://doi.org/10.1134/S1070427217010172
Werner E, Bell J (1922) The preparation of methylguanidine, and of β, β-dimethylguanidine by the interaction of dicyandiamide, and methylammonium and dimethylammonium chlorides respectively. J Chem Soc Trans 121:1790–1795
Holman R. (2007) Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 13-20. PMID: 18613325
Jackson RA, Hawa ML, Jaspan JB, Sim BM, Disilvio L, Featherbe D, Kurtz AB (1997) Mechanism of metformin action inJackson RA, Hawa ML, Jaspan JB, Sim BM, Disilvio L, Featherbe D, Kurtz AB (1997) Mechanism of metformin action in non–insulin–dependent diabetes. Diabetes 6:632–640
Ramsdell JW , Grossman JA, Stephens JM, Botteman MF, Arocho R (1999) A short-term cost-of-treatment model for type 2 diabetes: comparison of glipizide gastrointestinal therapeutic system, metformin, and acarbose. Am J Manag Care 5:1007–24
Marmwar PA (2016) Modified release of metformin hydrochloride using ion exchange resin complex in floating mucoadhesive tablets. Asian Journal of Pharmaceutics 10:7–15. https://doi.org/10.22377/ajp.v10i1.523
Rebitski EP, Aranda P, Darder M, Carraro R, Ruiz-Hitzky E (2018) Intercalation of metformin into montmorillonite. Dalt Trans 47:3185–3192. https://doi.org/10.1039/C7DT04197G
Stage TB, Brosen K, Christensen MMH (2015) A comprehensive review of drug-drug interactions with metformin. Clin Pharmacokinet 54:811–824. https://doi.org/10.1007/s40262-015-0270-6
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50:81–98. https://doi.org/10.2165/11534750-000000000-00000
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes H-P, Giardino I, Brownlee M (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790. https://doi.org/10.1038/35008121
Afinogenov G.E., Panarin E.F. Antimicrobial polymers, St. Petersburg Hippocrates (1993) 263 p
Connors KA (1987) Binding constants: the measurement of molecular complex stability. John Wiley & Sons, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision B.01, Inc., Wallingford CT
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146–401. https://doi.org/10.1103/PhysRevLett.91.146401
Wilbrandt W (1952) Behrens methods for calculation of LD50. Arzneimittelforschung 2(11):501–503
Furman BL (2015) Streptozotocin-induced diabetic models in mice and rats. Current Protocols in Pharmacology, USA. https://doi.org/10.1002/0471141755.ph0547s70
Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–320. https://doi.org/10.1016/j.phrs.2005.05.004
Yorek MA (2016) Alternatives to the streptozotocin-diabetic rodent International Review of Neurobiology, 127:89–112 in Nigel A. Calcutt, Paul Fernyhough (eds) Controversies In Diabetic Neuropathy, Academic Press https://doi.org/10.1016/bs.irn.2016.03.002
Barrière DA, Noll C, Roussy G, Lizotte F, Kessai A, Kirby K, Belleville K, Beaudet N, Longpré J-M, Carpentier AC, Geraldes P, Sarret P (2018) Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep 8:424–441. https://doi.org/10.1038/s41598-017-18896-5
Antony PJ, Sivasankaran K, Ignacimuthu S, Abdullah Al-Dhabi N (2017) High fat diet-fed, streptozotocin-induced diabetic rat model: is it an ideal type 2 diabetic model. J Endocrinol Diabetes Res 3:100–115
Motaal AA, El-Askary H, Crockett S, Kunert O, Sakr B, Shaker S, Grigore A, Albulescu R, Bauer R (2015) Aldose reductase inhibition of a saponin-rich fraction and new furostanol saponin derivatives from Balanites aegyptiaca. Phytomedicine 22:829–836. https://doi.org/10.1016/j.phymed.2015.05.059
Lineweaver H, Burk D (1934) The Determination of Enzyme Dissociation Constants. J Am Chem Soc 56(3):658–666
Ignat’ev VM, Emel’yanova NS, Fadeeva NV, Kurmaz SV (2020) Quantum chemical modeling the structure of complexes of copolymer of N-vinylpyrrolidone and triethylene glycol dimethacrylate with metformin. Russ J Phys Chem 94:713–718
Lebedeva TL, Fel’dshtein MM, Kuptsov SA, Plate NA (2000) Structure of stable H-bonded poly(N-vinylpyrrolidone) – water complexes. Polym Sci A 42:989–1005
Benmessaoud I, Koutchoukali O, Bouhelassa M, Nouar A, Veesler S (2016) Solvent screening and crystal habit of metformin hydrochloride. J Cryst Growth 451:42–51. https://doi.org/10.1016/j.jcrysgro.2016.07.001
Mondal S, Samajdar RN, Mukherjee S, Bhattacharyya AJ, Bagchi B (2018) Unique features of metformin: a combined experimental, theoretical, and simulation study of its structure, dynamics, and interaction energetics with DNA grooves. J Phys Chem B 122:2227–2242. https://doi.org/10.1021/acs.jpcb.7b11928
Childs SL, Chyall LJ, Dunlap JT, Coates DA, Stahly BC, Stahly GP (2004) A Metastable polymorph of metformin hydrochloride: Isolation and characterization using capillary crystallization and thermal microscopy techniques. Cryst Growth Des 4:441–449. https://doi.org/10.1021/cg034243p
World Health Organization. Global report on diabetes (2016) 83 p. https://apps.who.int/iris/handle/10665/204871
Jeffcoate WJ, Harding KG (2003) Diabetic foot ulcers. Lancet 361:1545–1551. https://doi.org/10.1016/S0140-6736(03)13169-8
Burlingham BT, Widlanski TS (2003) An Intuitive Look at the Relationship of Ki and IC50: A More General Use for the Dixon Plot. J Chem Educ 80(2):214
Ehrig T, Bohren KM, Prendergast FG, Gabbay KH (1994) Mechanism of Aldose Reductase Inhibition: Binding of NADP+/NADPH and Alrestatin-like Inhibitors. Biochemistry 33(23):7157–7165
Acknowledgment
This work was performed in accordance with the state task АААА-А19-119041090087-4, АААА-А19-119071890015-6 and supported by the Presidium of the Russian Academy of Sciences Basic Research Program “Fundamentals of technology and the use of features of nanostructures in nature sciences”. The equipment of the Scientific and Educational Center of the Moscow State Educational Institution (Chernogolovka) was used.
Funding
This research was supported by the Presidium of the Russian Academy of Sciences Basic Research Program “Fundamentals of technology and the use of features of nanostructures in nature sciences” and carried out in the frame of the State Task No. registration AAAA-A19-119041090087–4, AAAA-A19-119071890015–6. The authors declare that they have no financial conflicts of interest.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology: Svetlana V. Kurmaz; synthesis and experimental investigation: N. V. Fadeeva, G. V. Shilov; experimental investigation: Y. V. Soldatova, I. I. Faingold; computer modeling: V. M. Ignat'ev, N. S. Emel'yanova; writing—original draft preparation: Svetlana V. Kurmaz; writing—review and editing: R. A. Kotelnikova; supervision: D. A. Poletaeva.
Corresponding author
Ethics declarations
Research involving human and animal participants
All the animal experiments were performed according to the compliance with the EU Directive 2010/63/EU for animal experiments and the Russian law regulating experiments on animals.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurmaz, S.V., Fadeeva, N.V., Soldatova, Y.V. et al. New complexes of metformin based on the copolymer of N-vinylpyrrolidone with triethylene glycol dimethacrylate and their activity in experimental type 2 diabetes mellitus. J Polym Res 28, 345 (2021). https://doi.org/10.1007/s10965-021-02684-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02684-x